May 23, 2019, by NCI Staff
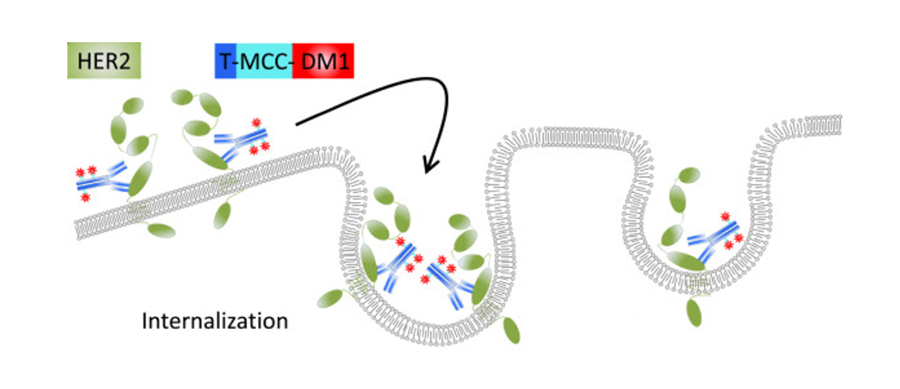
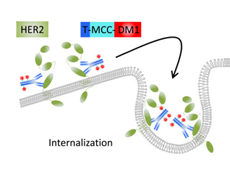
Once the antibody portion of T-DM1 binds to the HER2 receptor on cancer cells, emtansine is released into the cells.
Photo Credit: Adapted from Breast Can Res. 2014. doi: 10.1186/bcr3621. CC BY 2.0
The Food and Drug Administration (FDA) has expanded the approved use of the drug ado-trastuzumab emtansine (Kadcyla) to treat some women with HER2-positive breast cancer.
Ado-trastuzumab, also called T-DM1, was initially approved by FDA more than 6 years ago to treat women with metastatic HER2-positive breast cancer. Under the expanded approval, it can now be used when the cancer is far less advanced: as a post-surgical, or adjuvant, treatment in women with early-stage HER2-positive breast cancer. However, to be eligible to receive the drug under this newly approved use, women must first have undergone presurgical, or neoadjuvant, therapy to shrink their tumors and still have some signs of remaining invasive cancer, called residual cancer, in the breast or nearby lymph nodes.
The new approval, announced on May 3, is based on findings from a large clinical trial called KATHERINE that compared T-DM1 with trastuzumab (Herceptin) as an adjuvant treatment. In the trial, women treated with T-DM1 had a 50% reduced risk of their cancer returning or death than women treated with trastuzumab.
Side effects, including serious side effects, were more frequent in women treated with T-DM1. As a result, more women taking T-DM1 (29%) did not complete the full course of the adjuvant treatment than women taking trastuzumab (19%).
But many of these women did not have to stop taking the drug until they were near the end of their adjuvant treatment period, explained the study’s lead investigator, Charles Geyer, Jr., M.D., of the Virginia Commonwealth University Massey Cancer Center. In general, Dr. Geyer said, “the majority of women tolerated the drug reasonably well.”
The trial results, and the subsequent FDA approval, have already had an important impact on patient care, Dr. Geyer said.
“T-DM1 has now become the standard of care for women with HER2-positive breast cancer and residual invasive cancer following neoadjuvant therapy,” he said.
Building on Earlier Advances
Trastuzumab, a monoclonal antibody, was among the first FDA-approved targeted cancer therapies and has long been an established therapy for HER2-positive breast cancer. Trastuzumab latches on to HER2 proteins on the surface of breast cancer cells and prevents HER2 from stimulating cancer cell growth.
Known as an antibody–drug conjugate, T-DM1 chemically links the trastuzumab antibody to the chemotherapy drug emtansine (also known as DM1).
The antibody portion of T-DM1, in addition to blocking the activity of the HER2 protein on cancer cells, serves as a homing device for emtansine. Once the antibody binds to HER2 on cancer cells, emtansine is released into the cells.
After showing that T-DM1 improved how long women with metastatic HER2-positive breast cancer live, researchers quickly moved to test the drug in women with early-stage disease. The KATHERINE trial—funded by the manufacturer of T-DM1, Genentech—enrolled nearly 1,500 women with early-stage HER2-positive breast cancer, meaning their cancer was confined to the breast and the axillary lymph nodes. All women in the trial had evidence of residual disease after neoadjuvant therapy, which included chemotherapy and trastuzumab. Roughly 20% of the women also received pertuzumab (Perjeta) as part of their neoadjuvant therapy.
The goal of neoadjuvant therapy is to eliminate as much cancer as possible prior to surgery, and many women with early-stage HER2-positive breast cancer now receive neoadjuvant therapy, explained Janice Lyons, M.D., a radiation oncologist at the Case Comprehensive Cancer Center in Cleveland, who specializes in treating breast cancer. Some women with very small cancers, however, may proceed straight to surgery, she said.
For many women, neoadjuvant chemotherapy will eliminate all evidence of residual disease, Dr. Geyer said. Studies have consistently shown that women with early-stage breast cancer—particularly those with triple-negative or HER2-positive disease—who don’t have residual disease after neoadjuvant chemotherapy live longer without their disease recurring, compared with women who have residual invasive cancer.
Adjuvant therapy with trastuzumab has been a standard treatment for women with HER2-positive breast cancer, regardless of whether they have residual disease.
Participants in the KATHERINE trial were randomly assigned to receive adjuvant therapy with either T-DM1 or trastuzumab (in 3-week treatment cycles for up to 14 cycles).
The researchers who led the trial estimated that, at 3 years after beginning adjuvant treatment, 88% of women treated with T-DM1 were alive and free of invasive cancer, compared with 77% of women treated with trastuzumab.
It will take longer follow-up to determine whether T-DM1 will ultimately improve how long patients live overall, Dr. Geyer stressed.
“These results are impressive and clinically meaningful,” wrote Daniel F. Hayes, M.D., a breast cancer expert at the University of Michigan Rogel Cancer Center, in an editorial that accompanied the publication of the KATHERINE trial results last December in the New England Journal of Medicine. “Post-operative treatment with T-DM1 offers a major opportunity to improve long-term outcomes.”
Dr. Lyons agreed that adjuvant treatment with T-DM1 is the new standard of care for women with early-stage HER2-positive breast cancer who have residual invasive cancer after neoadjuvant chemotherapy. The “surprising result” from the KATHERINE trial, she said, is that all women benefited from T-DM1, “even those with very limited residual disease.”
Impact of Treatment Side Effects?
But these improvements, Dr. Hayes continued, do not come “without a price,” noting the higher rates of side effects and serious side effects. The latter included substantial drops in platelet levels and peripheral neuropathy.
Overall, 18% of women in the T-DM1 group stopped taking the drug because of specific side effects, compared with 2% of women in the trastuzumab group.
The increased side effects in women treated with T-DM1, Dr. Geyer noted, was most likely a cumulative effect of the pre- and post-surgical treatments. “Emtansine is a chemotherapy drug, and it was expected to add toxicity relative to no chemotherapy,” he said.
For some, lowering the dose of the drug alleviated side effects. Among the trial participants who eventually stopped taking T-DM1, some switched to trastuzumab for the remainder of the 14 cycles, which was a planned part of the study.
Dr. Lyons also noted that, in the KATHERINE trial, there was a slightly higher risk of lung inflammation (pneumonitis) in women who received T-DM1, although rates were low overall. This side effect was likely linked to radiation treatments those patients received. She advised clinicians to “carefully manage” the dose for any radiation treatment patients receive as part of adjuvant therapy with T-DM1.
Dr. Hayes advised against using T-DM1 in women without residual disease after neoadjuvant therapy or women with stage I disease at diagnosis. These patients “have a very favorable outcome” with the standard adjuvant therapy of paclitaxel and trastuzumab, he said.