October 3, 2018, by NCI Staff
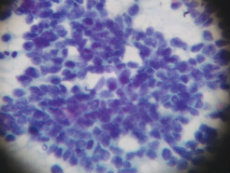
Small cell lung cancer cells
Credit: Clin Pract. 2012 May 29; 2(3): e62. CC BY-NC 3.0.
On March 18, 2019, the Food and Drug Administration approved atezolizumab (Tecentriq) for the initial treatment of patients with extensive-stage small-cell lung cancer. The approval, which covers the use of the drug in combination with the chemotherapy drugs carboplatin and etoposide, was based on the results of the IMpower133 clinical trial, described below.
For the first time in more than two decades, a treatment has been shown to improve how long patients with advanced small cell lung cancer (SCLC) live.
In a large clinical trial, treatment with the immunotherapy drug atezolizumab (Tecentriq), combined with a standard chemotherapy regimen, increased survival in patients with this highly aggressive form of lung cancer. Although the survival improvement was modest, the researchers hailed the positive survival findings as an important advance for the treatment of this intractable cancer.
“We’re really hoping that [these results are] just the beginning; that this is something we can build on further,” said the trial’s lead investigator, Stephen V. Liu, M.D., of Georgetown University’s Lombardi Comprehensive Cancer Center.
The results were presented on September 25 at the World Conference on Lung Cancer and published simultaneously in the New England Journal of Medicine.
Better Survival in SCLC: A Long Time Coming
Even compared with the more common form of lung cancer, non-small cell lung cancer, SCLC is particularly aggressive.
SCLC spreads very rapidly, often doubling the amount of tumor in the patient’s body in a matter of weeks, explained Frances Shepherd, M.D., a lung cancer researcher at Princess Margaret Cancer Centre in Toronto, during a conference press briefing. In fact, at the time of their diagnosis, most patients have what is called extensive-stage disease, Dr. Shepherd said, and surgery is not a treatment option.
All 400 patients in the trial—called IMpower133 and funded by the drug’s manufacturer, Genentech—had extensive-stage disease. Patients in the study were randomly assigned to either atezolizumab, an immune checkpoint inhibitor, in combination with standard chemotherapy (the drugs carboplatin and etoposide) or chemotherapy and a placebo.
The standard treatment actually shrinks tumors in most patients, Dr. Liu said. “But the response is transient,” he said. “We expect a response, we expect a relapse.”
Patients in the trial initially received atezolizumab and chemotherapy for four treatment cycles, called induction therapy, and then continued to receive atezolizumab or placebo alone after that, known as maintenance therapy.
Patients in the atezolizumab group lived longer overall: a median of 12.3 months, versus 10.3 months. The time it took for patients’ disease to begin progressing was also improved by approximately one month: a median of 5.2 months, versus 4.3 months.
Side effects related to treatment were seen in both patient groups, Dr. Liu said, with more immune-related side effects in patients treated with the checkpoint inhibitor. The most common serious side effects in patients treated with atezolizumab included anemia and reduced levels of white blood cells called neutropenia, which increases the risk of infection.
Importantly, Dr. Liu noted, treatment-related side effects did not prevent any patients from completing the induction therapy portion of the treatment.
A Small but Important Improvement
Although the overall survival increase in the trial was small, the fact that it was improved at all is a major achievement, Dr. Liu said.
Carboplatin and etoposide have been used to treat SCLC for more than 20 years, he said, because no other treatments have been able to help patients live longer.
“And it’s not for lack of trying,” Dr. Liu continued. More than 40 phase 3 clinical trials have been conducted during this time using more than 60 different drugs, he said, with none increasing survival.
Few patients diagnosed with SCLC survive for even a year despite treatment, said Joshua Bauml, M.D., of the University of Pennsylvania Abramson Cancer Center, who specializes in treating lung cancer but was not involved in the study. “So any advance in survival is really important.”
Some, however, were hoping for more from adding the immunotherapy drug to the standard treatment.
On Twitter, for example, Paul Wheatley-Price, M.D., of the University of Ottawa, called the finding “good news.” Even so, he added, “Greedily I want more benefit, but it’s a start.”
In addition to new therapies like atezolizumab, Dr. Bauml said, another potential avenue of further survival improvements in SCLC, which is strongly linked to smoking, may come from the increased uptake of lung cancer screening with chest CT. He pointed to the findings from another trial presented at the meeting, called NELSON, of screening of men and women at high risk of developing lung cancer because of their smoking history.
Using an approach of four screenings over more than 6 years or no invitation to screening, the trial—conducted in the Netherlands—showed an even larger decrease in lung cancer deaths than the NCI-funded National Lung Screening Trial.
The screening, noted Dr. Bauml, led to far more people being diagnosed with lung cancer at an earlier stage than they otherwise would have been without screening, meaning the cancers should be “potentially curable.” In one analysis from the trial, in fact, participants who underwent screening were far more likely to have surgery as a treatment for lung cancer than those were not invited for screening.
With “more robust screening programs,” Dr. Bauml continued, “we will see even more patients diagnosed with SCLC at an earlier stage.”