March 19, 2019, by NCI Staff
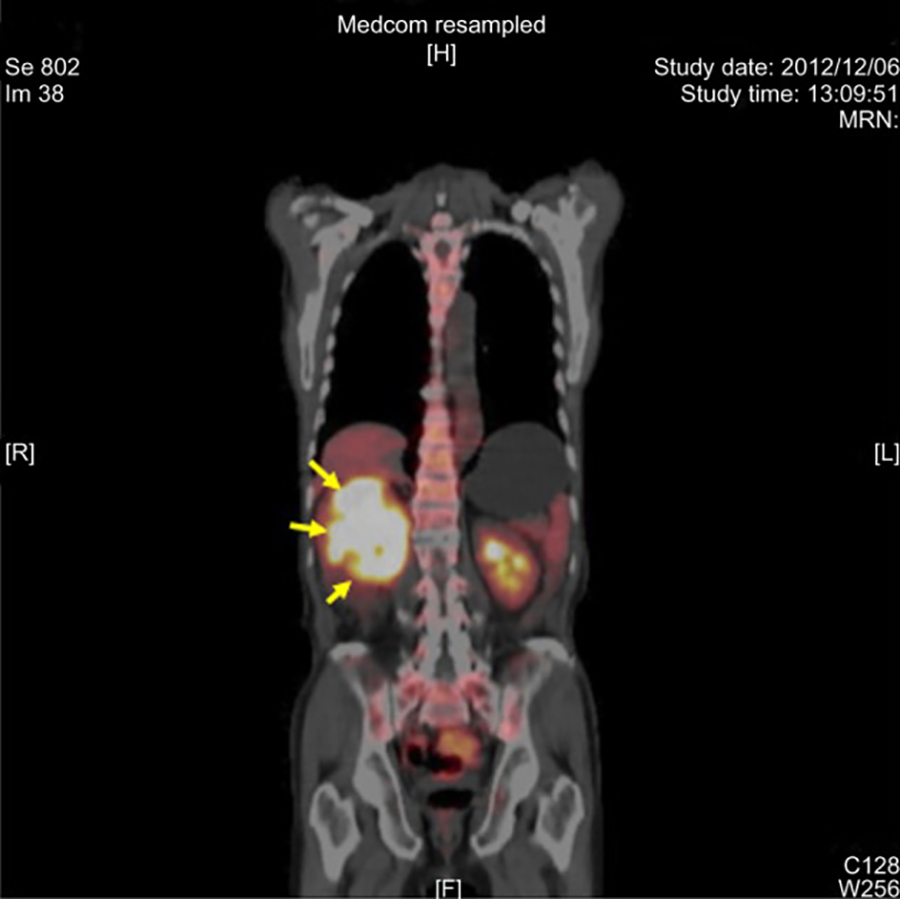
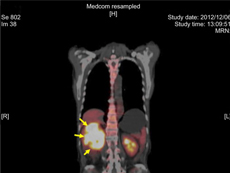
PET scan of a tumor in the right kidney (arrows).
Onco Targets Ther Feb 2014. doi: 10.2147/OTT.S58089. CC-BY 3.0
Results from two large clinical trials are expected to change the initial treatment for many people with newly diagnosed advanced kidney cancer.
In both studies, combination treatments that included a type of immunotherapy called an immune checkpoint inhibitor and the targeted therapy axitinib (Inlyta) led to better outcomes for patients with advanced kidney cancer than treatment with sunitinib (Sutent) alone, the standard of care for first-line therapy.
Results for the two phase 3 trials were reported last month at the American Society of Clinical Oncology Genitourinary Cancers Symposium (GU ASCO) in San Francisco and simultaneously published in the New England Journal of Medicine (NEJM).
The Food and Drug Administration (FDA) has already approved one immunotherapy combination as an initial, or first-line, treatment for people with advanced kidney cancer. And, based on these new data, several experts on the disease said further approvals for these patients are likely to be forthcoming.
“There’s a lot of exciting data. The treatment landscape is changing quickly,” said David McDermott, M.D., chief of medical oncology at Beth Israel Deaconess Medical Center, who has been involved in kidney cancer immunotherapy studies.
Evolving Treatment Strategies for Advanced Renal-Cell Cancer
In the last 12 years, the treatment of metastatic renal-cell cancer “has been revolutionized twice,” wrote Bernard Escudier, M.D., of the Gustave Roussy Cancer Campus in France, in an editorial accompanying the two papers in NEJM.
The first big change occurred more than a decade ago with the advent of targeted therapy. Sunitinib and, later, axitinib and similar drugs were developed that block a protein called the vascular endothelial growth factor receptor (VEGFR), which plays an important role in kidney cancer.
These targeted treatments were shown to be more effective than what was then the first-line treatment, interferon alpha, a treatment that worked only in a small number of patients and had serious side effects.
A second big change came in 2015, Dr. Escudier wrote, when the immune checkpoint inhibitor nivolumab (Opdivo) was shown to be effective in patients with advanced kidney cancer that had progressed after their initial treatment, soon after becoming the standard of care for these patients.
In 2018, FDA approved the combination of nivolumab and ipilimumab (Yervoy), two immune checkpoint inhibitors, as an initial treatment for the disease, after a large clinical trial showed that the approach led to better survival compared with patients treated with sunitinib.
Combining Targeted Therapy and Immunotherapy
The new trials took a different approach, combining the two strategies—a VEGFR inhibitor (axitinib) and an immune checkpoint inhibitor—that have been effective in treating kidney cancer on their own.
The studies used different immunotherapy agents but were otherwise very similar. Both trials enrolled more than 800 patients with previously untreated advanced clear-cell renal-cell carcinoma.
One study, funded primarily by Pfizer, tested the immune checkpoint inhibitor avelumab (Bavencio) in combination with axitinib, and showed that patients treated with the combination lived longer without their disease worsening (progression-free survival) compared with patients treated with sunitinib.
The second study, funded primarily by Merck, tested the immune checkpoint inhibitor pembrolizumab (Keytruda) along with axitinib and showed a significant improvement in how long participants lived overall (overall survival), as well as progression-free survival compared with sunitinib.
Toni Choueiri, M.D., of the Dana-Farber Cancer Institute, a senior author on the avelumab–axitinib study, explained, “We built the combination based on the fact that axitinib works in kidney cancer, and avelumab is in a class of agents that also work on kidney cancer. So you bring together two drugs that work.”
Earlier, smaller studies had shown that axitinib could be combined with either avelumab or pembrolizumab, all at full dose without causing unacceptable levels of toxicity in the liver. In contrast, combining sunitinib with an immune checkpoint inhibitor caused severe side effects, Dr. Choueiri said.
Axitinib targets VEGFR more specifically than sunitinib does, explained Brian Rini, M.D., of the Cleveland Clinic, a lead investigator on the pembrolizumab–axitinib study. “Axitinib is more potent and better tolerated, and made a better combination partner,” Dr. Rini said.
But some of “the most impressive data to date” for treating advanced kidney cancer are from the pembrolizumab–axitinib study, Dr. McDermott said.
The combination of pembrolizumab and axitinib “broadly out-performed sunitinib in all key [patient] groups,” said study investigator Thomas Powles, M.D., of the Barts Cancer Institute in London, in his presentation of the study results at the ASCO meeting. At a median follow-up of nearly 13 months, approximately 90% of patients treated with pembrolizumab and axitinib were still alive, compared with 78% of patients treated with sunitinib.
In the trial testing avelumab in combination with axitinib, patients treated with the drug combination had longer median progression-free survival (13.8 months versus 8.4 months) and were more likely to have their tumors shrink (objective response) compared with patients treated with sunitinib. At the time of publication, the patient follow-up was not long enough to determine whether there is an improvement in overall survival.
“This year we will assess overall survival,” Dr. Choueiri said.
Follow-up of patients in both studies will continue to monitor survival and long-term side effects, investigators from both trials said. Rates of side effects were high in all treatment groups in both trials, with high blood pressure and diarrhea being among the most common side effects.
The Shifting Treatment Landscape for Kidney Cancer
Combination treatments have “essentially been proven” to be superior to VEGFR inhibition alone as first-line therapy, Dr. McDermott said. Combination treatments have clear benefits, although they are more costly and cause more side effects, he added.
As for which combination treatment will be the best for which patients, that’s still unclear, Dr. Rini said. “There are subtleties. We don’t know the right answer yet,” Dr. Rini said. “We hope to sort this out over the next several years.”
“We now have three combination treatments positioned as first-line treatments,” said Robert Motzer, M.D., of Memorial Sloan Kettering Cancer Center, who was a lead investigator on the avelumab–axitinib study. Assuming these new combinations receive FDA approval, he expects that factors such as quality of life, safety, and the proportion of patients who experience complete responses will influence which treatments are used.
Before the advent of drugs like sunitinib, kidney cancer had a poor prognosis, with a median overall survival of less than a year, Dr. Motzer noted. With the use of VEGFR inhibitors, median survival improved to about 30 months.
And now immunotherapies are helping extend survival beyond 3 years, he said.
Treatments will continue to be given in sequence, he explained, as the existing treatments eventually stop working for most patients with advanced kidney cancer and their disease progresses.
In addition to studying different treatment sequences, researchers are also investigating whether they can identify features of tumors, or biomarkers, that can help predict which therapy might work better in different individuals, Dr. Motzer said.
“Ultimately, what’s most important to patients is the impact of these new approaches on long-term survival. How many patients have remissions? Are any patients actually cured of their kidney cancer?” Dr. McDermott said. “It’s going to take some time to figure those things out.”
Some of the new treatments for advanced kidney cancer are also being tested as adjuvant therapy in some patients diagnosed with less advanced disease who can be treated with surgery, he noted, especially those with stage III disease.
The adjuvant trials are currently enrolling patients, Dr. McDermott said, and he encouraged physicians and patients to consider joining the available trials.