May 15, 2019, by NCI Staff
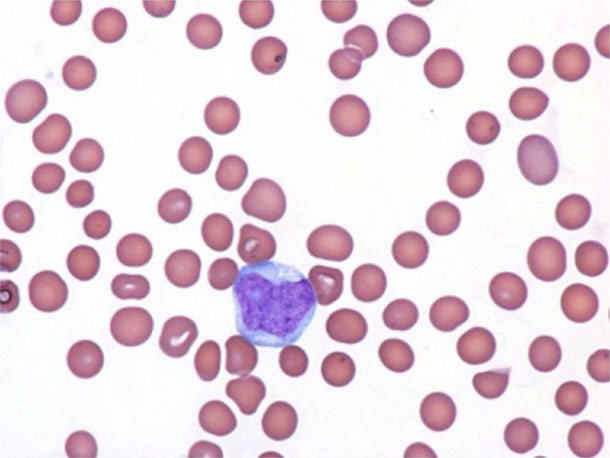
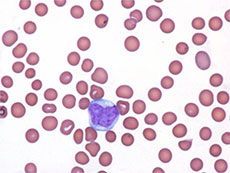
BPDCN cells (blue and purple) often form skin lesions but can also turn up in other places, like circulating blood (pink).
Credit: Clin Med Insights Case Rep 2013. doi: 10.4137/CCRep.S12608. CC-BY-NC 3.0.
Adults with a rare type of cancer called blastic plasmacytoid dendritic cell neoplasm (BPDCN) appear to benefit from treatment with tagraxofusp-erzs (Elzonris), according to findings from a clinical trial.
BPDCN is a rare, fast-growing blood cancer. Although it can develop at any age, it is most common among men in their late 60s. The usual survival for an adult is around 1 year.
Because there have been no standard treatments for BPDCN, doctors have relied on therapies that are typically used for other types of high-risk blood cancer, namely high-dose chemotherapy followed by stem cell transplantation. However, older patients are not usually able to tolerate this intensive approach, and even among younger patients, the cancer usually returns soon after treatment.
Guided by earlier results from this trial, as well as other trials, tagraxofusp was approved by the Food and Drug Administration (FDA) for the treatment of children and adults with BPDCN in December 2018, making it the first and only FDA-approved treatment for the disease.
The new findings, published April 25 in the New England Journal of Medicine, confirm and add to the preliminary results that led to last year’s approval, said lead investigator Andrew Lane, M.D., Ph.D., director of Dana-Farber Cancer Institute’s BPDCN Center.
Tagraxofusp reduced tumor burden in the large majority of patients, especially those who were newly diagnosed and had not received previous treatment for BPDCN.
“The advantage of this drug is that, when it produces a response, it appears to be long-lasting. That’s encouraging,” said Arthur Frankel, M.D., an oncologist at Mitchel Cancer Institute in Mobile, Alabama.
Dr. Frankel led the first clinical studies of tagraxofusp (then called SL-401) in people with BPDCN but was not involved in the current study.
What is BPDCN?
Up until 2008, BPDCN didn’t exist. That is, people had this type of cancer, but it was known by several different names and there weren’t clear parameters for how to define it. Even now, with a unified name and definition, BPDCN can be hard to diagnose because the disease is somewhat different in each patient.
For instance, most patients with BPDCN develop skin lesions—like a dark-colored rash or skin tumors—but cancer cells can also turn up in the bone marrow, lymph nodes, and circulating blood. Whereas some patients have lesions only in their skin, others have them in a combination of organs, Dr. Lane explained.
Another unusual characteristic of BPDCN is that it is three to four times more common in men than in women, but it’s not clear why.
In addition, a protein called CD123 is found at higher than normal levels on cancer cells from all patients with BPDCN and some patients with other types of blood cancer, making it an attractive drug target for blood cancer treatment.
“Researchers thought that BPDCN might be the poster child for targeting CD123,” Dr. Lane said. So, when tagraxofusp—a drug that attaches to CD123—became available, a clinical trial for people with BPDCN was the obvious first choice.
Tagraxofusp is a two-headed fusion protein that acts like a targeted missile. One part of it latches on to CD123 molecules on BPDCN cells, and, after the drug is absorbed by the cells, the other part—a toxin—kills the cells.
The Largest Study of BPDCN
The trial was conducted by researchers at nine institutions across the country who decided to work together after discovering a shared interest in BPDCN at the American Society of Hematology annual meeting in 2013.
“This [study] never could have been done at a single center because of the rarity of the disease,” Dr. Lane noted. “This is a truly collaborative effort between all these centers and the [pharmaceutical] company,” he added. The trial sponsor was Stemline Therapeutics, the manufacturer of tagraxofusp.
A total of 47 patients with BPDCN enrolled in the trial and received tagraxofusp. Fifteen of the participants had received previous treatment for BPDCN and 32 others had not.
The primary outcome of the study was the combined rates of complete response and clinical complete response. A complete response was defined as the disappearance of lesions in each organ (skin, bone marrow, and lymph nodes) where the cancer had appeared before treatment, and a clinical complete response was defined as a complete response with remaining skin discoloration that was not cancerous.
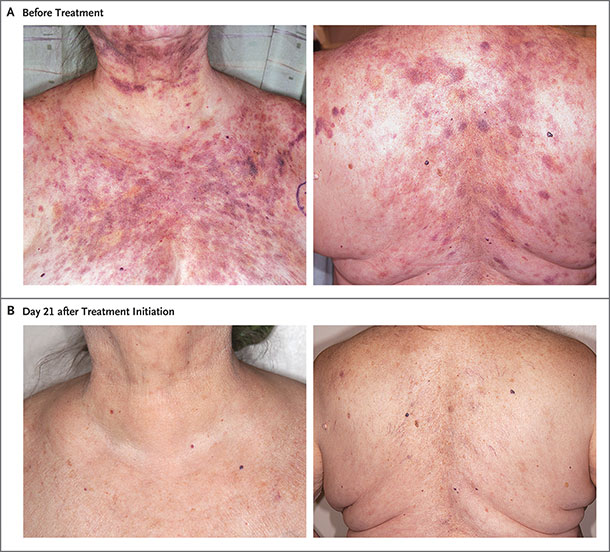
Photographs of a woman with BPDCN skin lesions before and after treatment with tagraxofusp.
Credit: The New England Journal of Medicine ©2019.
Among the 29 patients in the previously untreated group who were given a higher dose of tagraxofusp, the combined rate of complete responses and clinical complete responses was 72%. It’s not yet known how long these responses will last, but participants have so far been followed for a median of 19 months. The overall response rate (including complete responses, clinical complete responses, and partial responses) in this group was 90%.
Overall, 45% of patients in this group went on to receive a stem cell transplant, including older patients who ordinarily would have been “excluded from this intensive therapy,” the investigators noted. About half of patients in the previously untreated group (52%) were still alive 2 years after the start of the trial.
Among patients who had received previous treatment for BPDCN, 67% responded to tagraxofusp. The median length of response was 3 months. One patient in this group went on to receive a stem cell transplant.
Side Effects of Tagraxofusp
The most common side effects of tagraxofusp were an increase in liver enzymes, low albumin, swelling in the legs or other extremities, and low platelets. Serious adverse events were seen in about half of the patients.
Overall, 19% of participants experienced a potentially serious side effect called capillary leak syndrome, in which fluids and proteins leak out of blood vessels, resulting in dangerously low blood pressure. Two patients died as a result of this condition, leading the investigators to implement safety measures to prevent it, recognize early warning signs, and quickly treat symptoms.
“With vigilant monitoring and early interventions, capillary leak syndrome was manageable,” the study authors wrote. One additional death occurred after these measures were in place.
Capillary leak syndrome is also a side effect of other drugs that contain the same toxin as tagraxofusp, Dr. Lane noted. The FDA approval of tagraxofusp includes a warning and outlines factors that may increase the risk of capillary leak syndrome, such as abnormal heart function.
Remaining Questions about BPDCN
An important question that still needs to be answered is how many people develop BPDCN every year.
While some studies put the number at about 1,500 people per year, Dr. Frankel thinks that’s an underestimate. It’s likely that people with BPDCN are misdiagnosed because the disease is rare, looks different in each patient, and shares similarities with other blood cancers.
“We’re still in a period of increasing awareness of this disease,” Dr. Lane said. He added that he’s hopeful this study will make more oncologists aware of BPDCN and how to treat it.
Another question is whether other therapies can be combined with tagraxofusp to increase its efficacy in patients with BPDCN. Both Drs. Frankel and Lane have shown in lab studies that chemotherapy boosts tagraxofusp’s benefit for another kind of blood cancer called acute myeloid leukemia (AML).
Dr. Lane is leading a trial of tagraxofusp in combination with chemotherapy for people with AML or myelodysplastic syndrome and hopes a similar trial for people with BPDCN may soon be available.
Hope for Kids with BPDCN
Although BPDCN is rare in adults, it is extremely rare in children. But there are some similarities, and pediatric BPDCN cells also have higher-than-normal levels of CD123.
Consequently, doctors at City of Hope National Medical Center in Duarte, CA, have tested tagraxofusp as a treatment for children with BPDCN and reported three case studies last year.
Two of the three patients responded to the treatment, although the responses were short lived. One of these patients then went into remission after subsequently receiving chemotherapy and a stem cell transplant. The treatment was considered well tolerated and the side effects were manageable.
“We speculate that there might be underlying biological differences [in BPDCN] between children and adults, and additional studies with more patients are needed,” the investigators wrote.
Spreading Awareness with Social Media
Recognizing a lack of online resources for BPDCN patients, caregivers, and physicians, Dr. Lane and his colleagues began using social media to increase connections and share new information within the community. The disease-specific hashtag #BPDCN has been used on Twitter for the last 3 years and discussion and engagement continues to grow.