, by NCI Staff
More and more, people with lung cancer are receiving treatments that zero in on specific genetic changes in their tumors (targeted therapies). Now patients with non-small cell lung cancer (NSCLC) have another targeted therapy option.
On August 11, the Food and Drug Administration (FDA) gave accelerated approval to trastuzumab deruxtecan (Enhertu) for adults with NSCLC that has a certain kind of mutation in the HER2 gene (called an “activating” mutation). Around 3% of people with NSCLC have this kind of HER2 mutation.
To be eligible for treatment with Enhertu, patients must also have cancer that can’t be removed by surgery (unresectable) or has spread to other parts of the body (metastatic), and they must have already received one or more cancer therapies.
This approval is “highly anticipated and exciting,” said Joel Neal, M.D., Ph.D., a lung cancer doctor and researcher at the Stanford Cancer Institute.
“Up until this point, there were no FDA-approved targeted therapies for HER2-mutant lung cancer,” Dr. Neal added.
The approval was mainly based on the results of a phase 2 clinical trial, called DESTINY-Lung02, in which treatment with Enhertu shrank tumors of more than half of the study participants. All patients in the study had NSCLC that had gotten worse after receiving other treatments.
“After two decades of failed attempts in clinical trials, we have learned more about the biology of HER2 in lung cancer,” said, Bob T. Li, M.D., Ph.D., M.P.H., of Memorial Sloan Kettering Cancer Center.
Those lessons eventually led to the development and approval of Enhertu, said Dr. Li, who is the lead researcher of DESTINY-Lung02 and several other trials of Enhertu.
Under FDA’s accelerated approval, the companies that make Enhertu (AstraZeneca and Daiichi Sankyo) have to conduct another clinical trial to confirm the treatment’s benefits, such as improving how long patients live without their cancer getting worse.
Testing for HER2 mutations
Alongside Enhertu, FDA approved two companion diagnostic tests that check for HER2 gene mutations: Guardant360 CDx, which uses a blood sample, and Oncomine Dx Target Test, which uses a sample of tumor tissue.
This kind of genetic testing, often called biomarker testing, is “the critical first step” for treating people with lung cancer, Dr. Neal said.
People who have never smoked, are female, and are of Asian descent are more likely to have a HER2 mutation in their lung tumors, he noted.
But all patients with advanced NSCLC should get biomarker testing for this HER2 mutation and other mutations that have matching targeted therapies, Dr. Li said, such as those in the EGFR, ALK, and BRAF genes.
“Panel testing such as next-generation sequencing is now standard of care” for people with advanced lung cancer, he added.
The HER2 mutations seen in lung cancer make the HER2 protein activated all the time, Dr. Neal explained. That’s slightly different from breast cancer, in which tumors can have high levels of the HER2 protein from gene overexpression, he said.
Results of the DESTINY-Lung02 study
The DESTINY-Lung02 trial was conducted in multiple countries and included 102 patients. Half of the participants had never smoked, 69% were female, and 79% were Asian. All participants were randomly assigned to receive one of two doses of Enhertu, given as an infusion every 3 weeks.
Tumors shrank in 30 of 52 people (58%) who received the lower dose, including one person whose tumors disappeared completely. Among those whose tumors shrank, the treatment kept their cancer at bay for a median of 9 months.
Dr. Li and his team considered the lower dose to be optimal because it shrank tumors as well as the higher dose and caused fewer side effects. Enhertu is FDA-approved at the lower dose.
It’s also important to know how many participant’s tumors stayed the same size (called stable disease) while taking Enhertu, Dr. Neal said. As long as it isn’t causing too many side effects, doctors often keep patients on a lung cancer treatment even if their tumors are staying the same size or only shrinking slightly, he explained.
The percentage of people who had stable disease in DESTINY-Lung02 is not yet available. But in an earlier clinical trial of Enhertu for people with metastatic HER2-mutant NSCLC, 37% of the 91 participants had stable disease.
Side effects of Enhertu
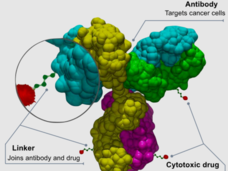
Enhertu is a type of drug known as an antibody–drug conjugate. The antibody portion binds to the HER2 protein on the surface of lung cancer cells. Then a chemotherapy drug that is tethered to the antibody slips inside the cancer cells and kills them.
“The drug part of it, the chemotherapy, is very potent. [So,] the side effect profile of this drug is mostly similar to chemotherapy,” Dr. Neal explained, such as nausea, hair loss, and low blood cell counts.
The side effects are mostly similar to standard chemotherapy, but may be less harsh because Enhertu is delivered directly to cancer cells, Dr. Li noted.
Enhertu also comes with a warning for a potentially deadly side effect called interstitial lung disease/pneumonitis. In studies of Enhertu in people with NSCLC and breast cancer, around 12% of patients experienced interstitial lung disease/pneumonitis.
That’s higher than what’s seen with other targeted therapies for lung cancer, Dr. Neal noted.
“Most of the time [interstitial lung disease/pneumonitis] is mild and reversible, but occasionally this could be severe and life-threatening,” Dr. Li said.
When the research team got better at detecting early symptoms of this condition and treating it (typically with steroids), this side effect became less severe, he added.
There are many ongoing studies of potential HER2-mutant lung cancer treatments that are very exciting, Dr. Li said. Some are looking at other antibody–drug conjugates that bind to HER2 or new small-molecule drugs that block the activity of HER2. If proven safe and effective, these drugs could potentially be used sequentially or combined with Enhertu, he said.
Capmatinib approved for MET-mutant lung cancer
On August 10, FDA gave full approval to capmatinib (Tabrecta) for treatment of metastatic NSCLC that has a mutation in the MET gene called “exon 14 skipping.” Around 3% to 4% of people with NSCLC have tumors with this type of gene mutation.
In 2020, FDA granted an accelerated approval to capmatinib for adults with this kind of cancer. The full approval is based on new study results confirming the treatment’s benefits.
According to the new findings, capmatinib shrank the tumors of 68% of patients who had not received any previous cancer treatment, and prevented tumors from growing back for a median of 17 months. Among those who had already received one or more cancer treatments, capmatinib shrank the tumors of 44% and kept them in check for about 10 months.
The most common side effects of capmatinib, which is taken as a pill twice a day, are swelling (edema), nausea, and pain. Capmatinib can also cause serious and potentially deadly side effects, namely interstitial lung disease/pneumonitis, liver toxicity, and pancreatic toxicity. It can also seriously harm unborn babies.
Another drug, called tepotinib (Tepmetko), is also FDA-approved for people with NSCLC that carries a MET exon 14 skipping mutation, Dr. Neal noted.