February 20, 2019, by NCI Staff
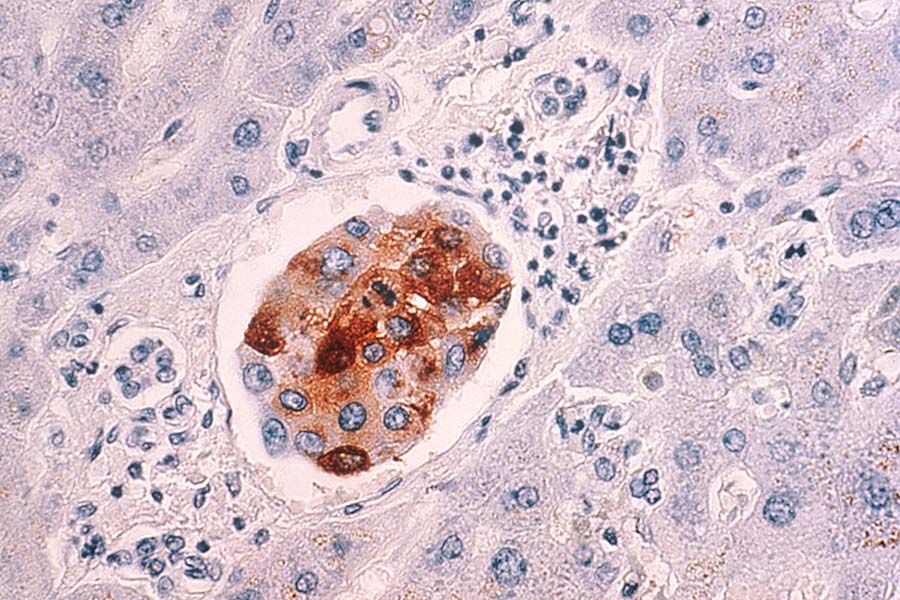
Metastatic breast cancer cells in the liver. A new approach aims to kill dormant cancer cells that have spread to other parts of the body.
Credit: National Cancer Institute
The phrase “hide and seek” likely brings to mind laughing children playing a harmless game. But it’s not fun and games when tumor cells hide. Tumor cells that have left the organ where they formed to hide elsewhere in the body can eventually emerge to produce metastatic disease. Killing these cells once they’ve hidden is extremely difficult.
The ability of escaped tumor cells to tuck themselves away poses a life-long risk of cancer recurrence for women with breast cancer and for patients with most other cancers.
“Very early during tumor progression, cancer cells leave the breast and [can] travel to the lymph nodes, bone marrow, liver, lungs, or brain,” said Cyrus Ghajar, Ph.D., of the Fred Hutchinson Cancer Research Center in Seattle.
These disseminated cells can remain inactive (that is, dormant or quiescent) for months, years, or even decades.
“In many cases, the original cancer itself is not the main problem—metastasis is,” said Nancy Boudreau, Ph.D., chief of the Tumor Metastasis Branch in NCI’s Division of Cancer Biology. “And these dormant metastatic cells are a bit like a time bomb; you just don’t know when they’re going to re-emerge again and start to grow.”
If cancer cells could be killed before they emerge from dormancy, the potential exists to stop metastatic tumors from ever forming. But most existing cancer treatments are thought to only target dividing cells, not dormant ones.
In a study published January 21 in Nature Cell Biology, a research team led by Dr. Ghajar showed in mice that, by disrupting the relationship between breast cancer cells that had spread to the bone and the normal cells surrounding them, they could make the hidden cancer cells sensitive to treatment. This was true whether the cancer cells were dividing or dormant.
“This is the first study to show that disseminated tumor cells, when they’re quiescent, can be sensitized to chemotherapy,” said Dr. Ghajar.
Disrupting the Tumor Cell Support System
The past two decades have brought an increasing awareness that the normal cells surrounding a tumor—called the tumor microenvironment—are important in cancer cell survival. So Dr. Ghajar and his colleagues wondered whether, when individual cancer cells take up residence in bone (a common location for breast cancer metastases), normal cells were somehow protecting the cancer cells from chemotherapy.
They started their work by figuring out exactly where in the bones breast cancer cells were hiding. In their initial experiments, they gave mice that had been implanted with mouse mammary cancer cells chemotherapy drugs commonly used to treat breast cancer.
When they examined bone samples from the chemotherapy-treated mice, the researchers found that most tumor cells that had survived were clustered around the blood vessels within the bone, an area called the perivascular niche.
In follow-up experiments, they used a type of 3-D cell culture that mimics the perivascular niche to further investigate whether normal cells there were somehow protecting the tumor cells and, if so, to identify which ones were doing so.
They found that endothelial cells lining the blood vessels seemed to be serving as the guardians of the tumor cells, protecting many of them from the three types of chemotherapy and one type of targeted therapy that the researchers tested.
Digging further, the researchers identified the molecules that appeared to help keep the tumor cells alive and safe through their interactions with the endothelial cells: integrins. These molecules play roles in normal blood vessel physiology and in wound healing, and they may be inadvertently helping tumor cells stay alive, explained Dr. Ghajar.
In their final experiments, the researchers used two antibodies that target the integrins they believed to be most important to the survival of tumor cells. In their 3-D cell culture system, treatment with these antibodies in combination with chemotherapy killed most tumor cells—including dormant cells—hiding in the perivascular niche. Neither the antibodies alone nor chemotherapy alone had as strong an effect.
When mice implanted with human breast cancer cells were treated with chemotherapy alone, almost 75% eventually developed metastases in the bones. But when given an anti-integrin antibody before chemotherapy, only 22% did.
No side effects besides those normally seen with chemotherapy were observed when the antibody was added to treatment—an unexpected and promising result, said Dr. Ghajar.
Exploiting a Window of Opportunity
The fact that the combination can kill dormant tumor cells “is a really good thing,” said Dr. Boudreau, who was not involved in the study. If an approach had to wake up disseminated tumor cells before it could kill them, that could potentially be dangerous, she explained: “Because then if you don’t get all the tumor cells [with treatment], you could get a population left behind that’s been induced to grow.”
There’s a long way to go before a combination of a drug to target integrins and chemotherapy could be tested in people, Dr. Ghajar cautioned. His lab has just started work to develop a version of the anti-integrin antibody that can be used in people. But the results from the current study “leaves us optimistic that this could work in humans without deleterious side effects, and without making [metastatic] disease worse,” he said.
They’re also trying to learn why almost a quarter of their mice still developed bone metastases, despite the combination therapy. The endothelial cells in the perivascular niche secrete different molecules that promote cell survival when exposed to chemotherapy, explained Dr. Ghajar. It’s possible that some tumor cells can also harness those molecules to escape the effects of treatment.
“We may need to target these other pathways in order to fully realize the ability of this type of therapy,” he said.
Whether or not similar mechanisms are at work in other types of cancer—and in other organs—remain to be seen, added Dr. Ghajar. He thinks they likely will, though the niches and molecules involved will almost definitely vary depending on the cancer type and organ. For example, while prostate cancer also spreads to the bones, those cells tend to hide near a type of bone-producing cell called osteoblasts instead of the endothelial cells, he explained.
But overall, the approach is worth pursuing, he continued. The period after tumor cells have spread but before they start to grow again “is a key window of opportunity, and one we’ve completely failed to take advantage of,” said Dr. Ghajar. “But it’s doable. We just have to figure out how exactly to make it work in patients.”