, by NCI Staff
The drug darolutamide (Nubeqa) could become part of the standard treatment for some men diagnosed with advanced prostate cancer, based on results from a large clinical trial.
In the trial, men with hormone-sensitive prostate cancer that had spread to other parts of the body, or metastasized, were treated with either darolutamide plus two other therapies, docetaxel and androgen deprivation therapy (ADT), or only docetaxel and ADT.
Substantially more men who received all three treatments were still alive 4 years after starting treatment than those treated with only docetaxel and ADT. And adding darolutamide didn’t lead to more intense side effects.
Data from the ARASENS trial were published in the New England Journal of Medicine and presented at the American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium on February 17.
“Based on the results of ARASENS, we conclude that darolutamide in combination with ADT and docetaxel should become a new standard of care for the treatment of patients with metastatic hormone-sensitive prostate cancer,” said the study’s lead investigator, Matthew R. Smith, M.D., Ph.D., of Massachusetts General Hospital Cancer Center, during the meeting.
Fatima Karzai, M.D., of NCI’s Genitourinary Malignancies Branch, agreed, calling the results “practice changing.”
ARASENS was funded by Bayer and Orion Pharma, the co-manufacturers of darolutamide. Bayer has submitted an application to the Food and Drug Administration to expand the drug’s approval to include people with metastatic hormone-sensitive prostate cancer. The drug is currently only approved for people with nonmetastatic prostate cancer that does not respond to hormone therapy (hormone-resistant).
An evolving standard of care
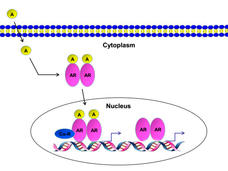
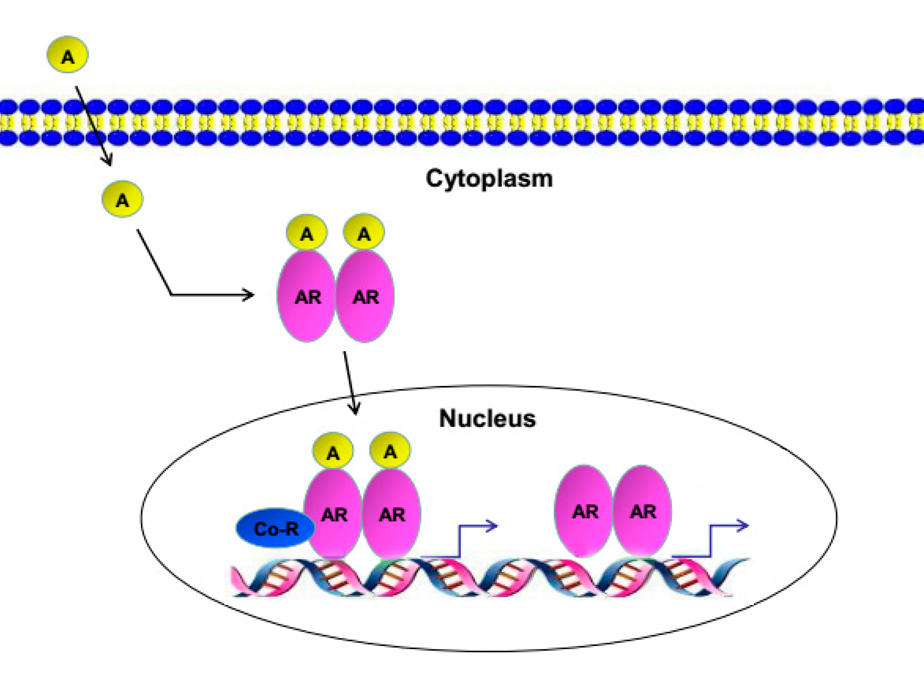
Hormone-sensitive (also called castration-sensitive) prostate cancer means a patient’s tumors are still largely being fueled by male sex hormones called androgens. For many years, metastatic hormone-sensitive prostate cancer was treated with ADT alone, which blocks the production of androgens by the testicles.
In 2014, a large clinical trial showed that adding the chemotherapy drug docetaxel to ADT improved survival in men with metastatic hormone-sensitive prostate cancer. Since then, this combination has become the standard of care for this group of patients.
More recently, studies have shown that adding other drugs that block the production or binding of androgens—including abiraterone (Zytiga), enzalutamide (Xtandi), and apalutamide (Erleada)—to ADT also helps people with metastatic hormone-sensitive prostate cancer live longer. In a trial combining apalutamide with ADT, for example, approximately 82% of men were still alive after 2 years compared with 74% of men treated with ADT alone.
Several clinical trials were then launched to see if combining any of these drugs with ADT and docetaxel could build on those survival gains. Results of those studies, however, have been mixed, with one showing an improvement in survival without the disease progressing and another finding no increase in overall survival.
Like other androgen receptor inhibitors, darolutamide works by blocking androgens from binding to receptors on cancer cells. Unlike other androgen receptor inhibitors, however, darolutamide does not cross from the bloodstream into the brain, which may be why studies have found fewer central nervous system–related side effects (e.g., seizures) with darolutamide than with other such drugs.
The trio of darolutamide, ADT, and docetaxel has already been shown to improve survival in men with hormone-resistant prostate cancer that has not spread. So ARASENS was launched to see if it could do the same in men with prostate cancer that has spread.
Improved survival at 4 years
In the ARASENS trial, nearly 1,300 participants were randomly assigned to receive darolutamide or a placebo (both taken as a pill, twice a day). All participants received ADT within 12 weeks before randomization and six cycles of docetaxel starting within 6 weeks after randomization.
After 4 years, about 63% of patients who received darolutamide were still alive compared with about 50% of patients who received placebo. The group that received darolutamide lived longer even though most participants in the placebo group (75%) received other commonly used treatments, including abiraterone and enzalutamide, during follow-up.
Darolutamide resulted in other improvements as well. For example, among those treated with darolutamide, the time for their cancers to become resistant to hormone-suppressing therapies was longer, as was the time until the pain caused by their cancer got worse.
The frequency of serious side effects—which included fatigue, falls, fractures, and cardiac issues—was similar in the two groups. Roughly two-thirds of the patients in both groups experienced serious side effects, most of which occurred when darolutamide (or placebo) were given at the same time as docetaxel.
More options lead to more questions
Elisabeth Heath, M.D., director of prostate cancer research at Karmanos Cancer Institute in Detroit, agreed that the ARASENS results should have an immediate impact on how this form of the disease is treated.
Speaking at the ASCO symposium, Dr. Heath, who was not involved in the study, highlighted an important difference between ARASENS and other trials that tested androgen receptor–blocking drugs in men with this form of prostate cancer. In those other trials, she explained, some participants received docetaxel prior to treatment with the androgen receptor–blocking drugs rather than at the same time.
Based on the ARASENS results, Dr. Heath said, giving all three treatments simultaneously looks to be the preferred option for some patients.
Dr. Karzai noted that despite there being multiple options to treat metastatic hormone-sensitive prostate cancer, many questions remain. “We don’t have guidelines on who should start with what drug and whether one drug is better than another for a [specific] patient,” she said.
She also pointed out that more research is needed on how the order in which the drugs are given impact their effectiveness and the frequency of side effects.
Additionally, she said, the survival improvement in the ARASENS trial was seen in patients whose cancer had spread in multiple areas beyond the prostate (known as high-volume disease).
“We don’t know if people with lower-volume [disease would] benefit from [the addition of darolutamide] as much as the patients with higher-volume disease do,” she said.
“You have to really think about [the group with lower-volume disease]. Do you want to give them this therapy that causes side effects when you don’t know if they are going to get the survival benefit and the other secondary benefits like the high-volume group does?” she said.
Dr. Smith said that future studies could look at whether darolutamide and ADT alone could improve survival as well as darolutamide, ADT, and docetaxel. Removing docetaxel from the combination could reduce some of the side effects, he said.