It’s often said that cancer is a disease of ageing.
And there’s a reason for that. Cancer starts with small faults in our DNA, called mutations. These mutations are usually fixed by our bodies, but sometimes they can slip through the net and begin to build up. If a cell accumulates enough mutations, it may begin to divide and multiply uncontrollably.
That means over time, more and more mutations can build up, with each one making a cell more likely to become cancerous.
It’s reflected in the statistics too. Half of all cancers are diagnosed in people over 70, and incidence rates for all cancers combined are highest in the 85-89 age bracket.
However, the stories about cancer that tend to make headlines are in younger people because they’re the most shocking. But this doesn’t accurately reflect the population most likely to get cancer. In fact, getting cancer at a young age is relatively rare.
This often leaves ageing as the ‘forgotten’ cancer risk factor, overshadowed by preventable risk factors like smoking and obesity.
And while accumulated damage to our DNA is a big factor in the increased risk of cancer as we age, it alone is not enough to explain the stark increase in cancer incidence we see in the elderly population. To get the full picture, we need to investigate the other factors at play here.
“A completely different cancer”
We know that cancer biology differs in older adults, but there is no one change to our cells associated with ageing that accounts for the increased likelihood of developing different types of cancer. There are different processes associated with ageing impacting cancer initiation in different parts of the body.
</p>
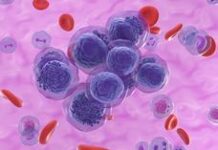
<p> ” data-medium-file=”https://news.cancerresearchuk.org/wp-content/uploads/2023/05/CLL-age-1.jpg” data-large-file=”https://news.cancerresearchuk.org/wp-content/uploads/2023/05/CLL-age-1.jpg” decoding=”async” class=” size-full wp-image-25715″ src=”https://news.cancerresearchuk.org/wp-content/uploads/2023/05/CLL-age-1.jpg” alt=”A blood smear showing the presence of chronic lymphocytic leukaemia cells” width=”200″ height=”200″ srcset=”https://news.cancerresearchuk.org/wp-content/uploads/2023/05/CLL-age-1.jpg 200w, https://news.cancerresearchuk.org/wp-content/uploads/2023/05/CLL-age-1-150×150.jpg 150w, https://news.cancerresearchuk.org/wp-content/uploads/2023/05/CLL-age-1-96×96.jpg 96w” sizes=”(max-width: 200px) 100vw, 200px”></p>
<p class=) A blood smear showing the presence of chronic lymphocytic leukaemia cells (dark purple) Jose Luis Calvo/Shutterstock
A blood smear showing the presence of chronic lymphocytic leukaemia cells (dark purple) Jose Luis Calvo/Shutterstock
Take leukaemia, for example, a type of blood cancer starting in white blood cells.
Leukaemia incidence is strongly related to age, with the highest incidence rates being in older people. In the UK in 2016-2018, on average each year almost 4 in 10 new cases (38%) were in people aged 75 and over.
“One of the fascinating things in the blood system is the cancer that you get when you’re young is completely different to the cancer you get when you’re old,” says Professor David Kent. “And the origins of most blood cancers are traceable back to blood stem cells.”
Kent is a research group leader at the York Biomedical Research Institute at the University of York.
His laboratory research focuses on understanding normal blood stem cells and how changes in their regulation lead to cancers like leukaemia.
Childhood leukaemia is the result of, as Kent describes it, ‘cells not knowing when to stop’. As the body starts growing, cells need to adapt to replicate at the correct rate. If it starts producing too many cells, in this case blood cells, that’s when cancer can start.
But in the elderly, it’s a very different story.
A shift in pressure
“One of the things that can happen during ageing is a lower production of the various mature blood cell types that you normally have,” Kent explains.
“For example, age-related anaemia, which is a lack of red blood cells, is typically considered to be the result of an ageing bone marrow.”
Bone marrow is a tissue found in the ‘spongy’ areas of your bones. It’s where your body generates new blood cells from blood stem cells.
But a lack of mature cells can create another problem – it can put more pressure on the immature stem cells to produce that type of cell to make up for the deficiency.
“Whenever you put that sort of proliferative or external stress on a cell, it has a chance, albeit a very small chance, of acquiring or selecting for genetic mutations, and potentially becoming cancerous.”
And this pressure to produce more cells isn’t the only stressor these blood stem cells have to contend with.
“There’s a really interesting field now emerging, inflammation related ageing, commonly referred to as ‘inflammageing’.
“It suggests that as we age, we have changes in the environment where the blood stem cells live.
“As we get older, these inflammatory molecules, proteins that are best known for being produced when you have infections, become elevated and this has the potential to place a selection pressure on particular stem cells that have or don’t have particular mutations.”
This changing environment can therefore cause stem cells carrying mutations to proliferate where ‘normal’ cells would have previously, potentially leading to cancer.
“If these mutated cells are going to grow out, the big question is, how do we stop them? And how do we change those selective pressures? I think that’s going to be a fascinating area of future research.”
The view from the clinic
Blood cancers are an example of liquid cancer, meaning the tumour doesn’t form into a solid mass. But let’s delve into a solid cancer.
 in situ shown under the microscope. </p>
<p> ” data-medium-file=”https://news.cancerresearchuk.org/wp-content/uploads/2023/05/melanoma-age-1.jpg” data-large-file=”https://news.cancerresearchuk.org/wp-content/uploads/2023/05/melanoma-age-1.jpg” decoding=”async” loading=”lazy” class=” size-full wp-image-25716″ src=”https://news.cancerresearchuk.org/wp-content/uploads/2023/05/melanoma-age-1.jpg” alt=”Melanoma cells in situ shown under the microscope. The melanoma cells have been stained red” width=”200″ height=”200″ srcset=”https://news.cancerresearchuk.org/wp-content/uploads/2023/05/melanoma-age-1.jpg 200w, https://news.cancerresearchuk.org/wp-content/uploads/2023/05/melanoma-age-1-150×150.jpg 150w, https://news.cancerresearchuk.org/wp-content/uploads/2023/05/melanoma-age-1-96×96.jpg 96w” sizes=”(max-width: 200px) 100vw, 200px”></p>
<p class=) Melanoma cells (stained red) in situ shown under the microscope. Lisa Culton/Shutterstock
Melanoma cells (stained red) in situ shown under the microscope. Lisa Culton/Shutterstock
Incidence rates for melanoma skin cancer in the UK are highest in people aged 85 to 89 and each year more than a quarter (29%) of all new melanoma skin cancer cases in the UK are diagnosed in people aged 75 and over (2016-2018).
Treating melanoma, and cancer in general, is a unique challenge in the elderly. One that Dr Amaya Virós is very familiar with.
Virós is a researcher at our Manchester Institute, alongside her work as a clinical dermatologist at Salford Royal Foundation Trust in Manchester. Her lab focuses on how ageing influences the initiation and progression of melanoma.
Her work makes her both an expert in the biology of melanoma and treating it in the clinic.
“The processes that drive cancer forward often overlap with the mechanisms that occur physiologically during ageing,” she says.
“Melanoma in the elderly is more common in sites that have been chronically sun exposed, like the head and neck, in contrast to melanoma in younger individuals where you have melanomas arising on skin that is only exposed during holidays, like the trunk.”
But the site of the cancer on the body isn’t the only difference between the young and old.
“Having multiple skin cancers is a huge clinical challenge and concern, because if you get melanoma at an older age, you’re very likely to develop other skin tumours like squamous or basal cell carcinomas.”
“Age can increase your risk of having other cancers as well, prostate or breast for example. All those high incidence cancers also go hand in hand with age.”
And if that weren’t enough, there are many factors uniquely affecting older adults that can impact on their eligibility for certain treatments, or how successful treatment may be. For example, some patients may have additional health problems, or take other medications that preclude them from receiving the preferred cancer treatments.
“We’re frequently looking in clinical practice at patients who have lots of comorbidities and complications, the chronic patients. I never discharge a patient who’s over 70 with skin cancer and severely sun damaged skin, because they just keep coming back.”
Targeting the right population
What Virós sees through her practice in the clinic presents a clear message: we need to account for elderly patients much earlier in the treatment pathway. In fact, we need to be thinking about them when we’re developing treatments.
“The problem is that the models we’ve used so far don’t account for age. We’re using young mice, young cells, using cancer cell lines without considering the effect of an aged environment on a cancer cell for example.
“There’s no standardised, age stratified treatments. And age is always analysed in hindsight in clinical trials. In fact, I think we should start building clinical trials stratifying patients by age.”
And our shift in perspective needs to span the entire cancer pathway, from research to diagnosis. That includes raising awareness of who’s most at risk of developing melanoma.
“Media campaigns are currently not targeting the population that is at highest risk for melanoma. Campaigns frequently use photographs of young patients to raise awareness of skin cancer, and of course young people can die of melanoma, but that isn’t the typical melanoma patient. The typical patient is over 55 years old.”
“My advice to GPs: if you see an elderly patient come in for hypertension, use the opportunity to check their skin. GPs frequently don’t have the time, but that’s the best way you catch skin cancer early. And that’s how you prevent death.”
A grand challenge
Research like Kent’s is showing us just how complex the relationship between cancer and ageing is, and Virós’s practice in the clinic demonstrates just how urgently we need to develop more effective treatment for the elderly.
And the scale of this issue is only set to grow. By 2045, it is predicted that the number of people in the UK aged 85 and over will reach 3.1 million. That’s almost double the 1.7 million in 2020. And an ageing population unfortunately means an increasing number of cancer diagnoses.
So, if we’re going to uncover the mysteries of what specific cellular changes cause an increased risk of cancer across different tissues as we age, the time to do it is now.
But that will be no mean feat. In fact, you could say it’s a grand challenge.
Cancer Grand Challenges, a global funding initiative co-founded by Cancer Research UK and the National Cancer Institute in the US, think so too.
That’s why they are inviting interdisciplinary teams from across the globe to apply for up to £20 million in funding to take on 9 recently announced challenges – each representing some of the biggest questions in cancer research.
One of these new challenges is to gain a thorough understanding of the cellular and immune changes associated with ageing and how they contribute to cancer risk in different organs, bringing the ‘forgotten’ cancer risk factor into the spotlight.
“Cancer Grand Challenges is setting the agenda for researchers across the world to come together and make change,” said Dr David Scott, the Director of Cancer Grand Challenges. “Our initiative inspires new thinking – bringing together world-class, multidisciplinary teams to find bold, new solutions to cancer’s most complex problems.”
We’ve seen the impact that a Cancer Grand Challenges team can have on the big questions they set out to tackle in the past. To name just a couple, the IMAXT team has created an entirely new way to visualise a tumour, and the eDyNAmiC team is showing how tiny rings of DNA, called extrachromosomal DNA, could be the key to how cancer evolves to evade treatment.
Now, if we can uncover the specific biological changes in individual organs that increase the risk of developing cancer as we age, we can put the elderly in the spotlight when we’re developing treatments.
In doing that, we can find new strategies to decrease cancer risk in ageing populations.
Jacob