September 4, 2018, by NCI Staff
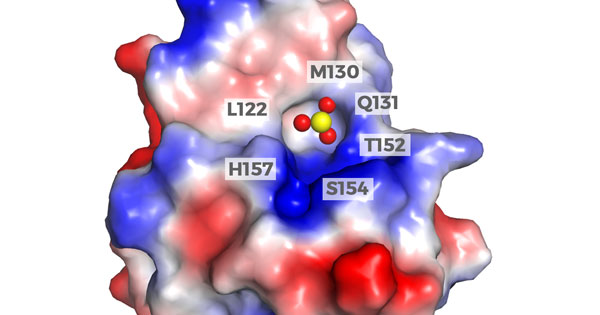
A model representing the crystal structure of arsenic (red and yellow spheres) interacting with and inhibiting the activity of the Pin1 protein (red, white, and blue).
Nature Communications Aug 2018 doi: 10.1038/s41467-018-05402-2. CC BY 4.0.
A two-drug combination commonly used to treat a type of leukemia blocks an enzyme that has a central role in breast and many other cancers, a new study has found.
The drug combination, arsenic trioxide (Trisenox) and tretinoin (also known as retinoic acid), has essentially turned acute promyelocytic leukemia (APL) from a fatal disease into a curable one. But the mechanism by which it kills cancer cells has been a mystery.
In an earlier study, Kun Ping Lu, M.D., Ph.D., and Xiao Zhen Zhou, M.D., of Beth Israel Deaconess Medical Center, in Boston, and their colleagues found that retinoic acid inhibits the key enzyme, Pin1. Now, they’ve found that arsenic also blocks Pin1, and the combination of the two drugs inhibits the enzyme more effectively than either drug alone.
Pin1 is considered a cancer master regulator—a protein that regulates entire cellular networks that are critical to cancer progression. Some scientists consider master regulators ideal drug targets.
The findings are “an interesting launching pad for further investigation,” said Joanna Watson, Ph.D., of NCI’s Division of Cancer Biology, which helped fund the study.
The combination treatment slowed the growth of several different laboratory models of breast cancer, the researchers reported. Their findings, published August 9 in Nature Communications, provide the rationale to test the drug combination in a clinical trial of women with breast cancer, said Dr. Lu.
Pin1: An Ideal Drug Target
Pin1 switches the shape of the amino acid proline within proteins. This conformation change can activate 40 proteins that promote tumor growth and inactivate more than 20 proteins that suppress tumor growth.
Pin1 is overactive in many different types of cancer, including breast, lung, prostate, and colon cancer. It is also found at higher than normal levels in breast cancer stem cells—specialized cancer cells that are thought to be responsible for tumor initiation, growth, metastasis, and drug resistance.
On the other hand, mice that lack Pin1 are protected from developing cancer even in the presence of cancer-causing mutations in other genes.
Because arsenic is often combined with retinoic acid to treat APL, the researchers wanted to know if arsenic also targets Pin1. But rather than studying APL, they focused on triple-negative breast cancer because this aggressive disease has a poor prognosis and is not effectively treated with targeted therapies.
Arsenic and Retinoic Acid Work Together
First, the researchers found that arsenic inhibited Pin1 activity, which in turn slowed the growth of triple-negative breast cancer cells. They then showed that arsenic needed to bind to Pin1 to impede breast cancer cell growth. Interacting with arsenic makes Pin1 unstable, explained Dr. Lu, and as a result, the protein is destroyed.
Arsenic also lowered Pin1 levels and slowed the growth of breast cancer xenograft tumors in mice.
Next, the researchers examined the effects of combining arsenic and retinoic acid in cell and mouse models of triple-negative breast cancer.
Treating cells or mice with arsenic or retinoic acid alone lowered Pin1 levels and slowed cell growth. But the combination of the two drugs had an even greater effect. The combination also prevented Pin1 from altering the levels of certain proteins that control cancer growth, producing effects that are similar to those of inactivating the Pin1 gene in breast cancer cells.
The combination showed synergy, meaning that the resulting effect “is above and beyond what you would expect by just adding the two [drugs] together,” explained Dr. Watson.
Synergy may reflect the fact that, in APL cells, retinoic acid treatment raises the level of a transporter that brings arsenic inside of cells. Levels of the transporter also increased when Dr. Lu and his colleagues treated triple-negative breast cancer cells with retinoic acid. By contrast, arsenic and retinoic acid did not act synergistically in triple-negative breast cancer cells engineered to lack this transporter.
These results suggest that, in addition to inhibiting Pin1 directly, retinoic acid increases the amount of arsenic in cells by boosting the level of this transporter, Dr. Lu explained. The arsenic then further inhibits Pin1.
He added, this also implies that when used in combination with retinoic acid, a lower concentration of arsenic could achieve the desired effect—potentially reducing the side effects of arsenic, which is extremely toxic at high doses.
Targeting Breast Cancer Stem Cells
Developing drugs that effectively target cancer stem cells has proven to be a challenge, and cancer stem cells that remain after treatment are thought to be a cause of cancer relapse and metastasis.
Dr. Lu’s team found that, to a greater extent than either drug alone, the combination of arsenic and retinoic acid eliminated triple-negative breast cancer stem cells. The effects of the combination treatment were similar to those of inactivating the Pin1 gene in breast cancer cells, they found.
The researchers also found that the combination of arsenic and retinoic acid eliminated cancer stem cells that were resistant to chemotherapy, and it eliminated cancer stem cells in mouse models of triple negative breast cancer.
New Insights and Potential New Therapy
It had long been thought that arsenic’s main target was a fusion protein that is found only in APL, and there were doubts that the drug would be effective against other types of cancer, despite some evidence of activity. In their earlier study, Drs. Lu and Zhou and their colleagues found that retinoic acid targets this fusion protein by inhibiting Pin1.
“Identifying Pin1 as the major target of arsenic will hopefully stimulate clinical trials for other [types of] cancer,” Dr. Lu said.
The researchers hope to develop a clinical trial to combine arsenic with the standard therapy for women with triple-negative breast cancer. And because their findings show that arsenic’s activity depends on the expression of Pin1 and its transporter, Dr. Lu said they want to select patients whose cancers express both proteins.
As for retinoic acid, it is normally broken down quickly inside the human body. To enhance its ability to reach solid tumors, the team is interested in developing a more stable form of retinoic acid. If such a form can be created, it could potentially be combined with arsenic in clinical trials, Dr. Lu said.
Another advantage of having learned one mechanism of arsenic’s anticancer activity, said Dr. Watson, is being able to develop new drugs that potentially target Pin1 more efficiently and with fewer side effects than arsenic itself. The research team is also working to develop a targeted drug that blocks Pin1 activity.
Dr. Lu noted that because Pin1 controls multiple pathways that contribute to cancer development, cancer cells might be less able to develop resistance to drugs that block Pin1’s activity—something that often occurs with targeted therapies that attack a single pathway.








