October 15, 2018, by NCI Staff
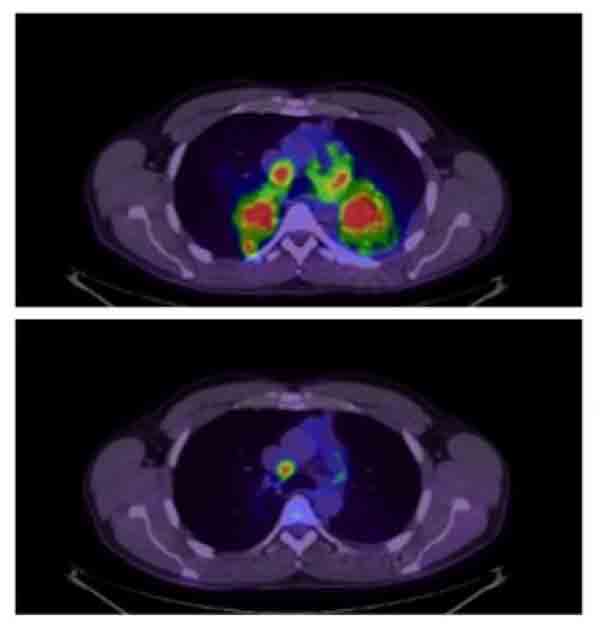
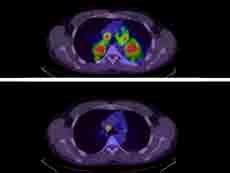
In patients with non-small cell lung cancer whose tumors have alterations in the ALK gene, ALK-inhibitors like brigatinib and crizotinib can dramatically shrink tumors.
Credit: J Pers Med April 2012. CC BY 3.0.
New results from two large clinical trials are expected to have an immediate impact on the care of patients with the most common form of lung cancer.
The drugs tested in the trials, brigatinib (Alunbrig) and durvalumab (Imfinzi), respectively, are already being used to treat some patients with the disease, non-small cell lung cancer (NSCLC). Lung cancer experts agreed that the trials’ findings should further cement the value of these drugs in treating NSCLC, and continue an important trend.
“We’re seeing a lot of changes in the treatment of non-small cell lung cancer,” said Azam Ghafoor, M.D., of NCI’s Thoracic and GI Malignancies Branch. “In particular there has been a paradigm shift in the expanded use of immunotherapy and targeted therapies.”
Results from both trials were presented September 25 at the World Conference on Lung Cancer in Toronto and simultaneously published in the New England Journal of Medicine.
For NSCLC with ALK Alterations, Multiple Options
Brigatinib is an inhibitor of the ALK protein, the gene for which is altered (most commonly as a fusion, or translocation, with another gene) in 4%–7% of patients with NSCLC. It was initially approved by the Food and Drug Administration (FDA) last year to treat patients with NSCLC whose tumors have an ALK alteration and whose cancer has progressed despite treatment with crizotinib (Xalkori). In 2011, crizotinib became the first ALK-targeted drug to receive FDA approval.
The ALTA-1L trial compared the drug against crizotinib in people with advanced NSCLC. The trial was funded by Takeda Pharmaceutical Company, the manufacturer of brigatinib.
In the trial, patients who were treated with brigatinib lived longer without their disease worsening than those treated with crizotinib. At 12 months after initiating treatment, 67% of patients treated with brigatinib were still alive without any evidence of their cancer getting worse, compared with 43% of those treated with crizotinib.
Brigatinib was also more effective than crizotinib at shrinking brain lesions among trial participants whose cancer had spread to their brain: 78% of patients treated with brigatinib, compared with 29% of patients who received crizotinib.
The latter finding was not necessarily a surprise to researchers. As a second-generation ALK inhibitor, brigatinib was designed to address some of the shortcomings of the first-generation crizotinib. Among the improvements in the second-generation ALK-targeted drugs—which also includes alectinib (Alecensa) and ceritinib (Zykadia)—is an enhanced ability to cross the blood–brain barrier.
That particular improvement is critical because, in NSCLC with ALK alterations, “[disease] progression tends to occur in the brain,” explained the trial’s lead investigator, Ross Camidge, M.D., of the University of Colorado Cancer Center, during a conference press briefing.
Alectinib has already been approved by FDA as an initial treatment for patients with ALK-positive tumors, based on findings from a phase 3 clinical trial in which it extended progression-free survival longer than crizotinib and was more effective at preventing and shrinking brain metastases.
Both brigatinib and alectinib have side effects, but they are not entirely the same. In the ALTA-1L trial, for example, a small percentage of patients treated with brigatinib experienced inflammation in the lungs, a problem that has not been seen with alectinib.
Dr. Camidge noted that, overall, patients tolerated brigatinib very well. He also noted that the trial included patients who had already received chemotherapy—which the alectinib versus crizotinib trial did not—so ALTA-1L participants were more representative of a real-world population of patients.
Brigatinib is not yet approved by FDA as an initial treatment in patients with ALK-positive NSCLC. But if that happens, Ravi Salgia, M.D., Ph.D., associate director for clinical sciences at the City of Hope Cancer Center, said that either alectinib or brigatinib could be the preferred first choice in this group of patients.
“That’s what medical oncology is. You use what you have the most experience with and then gain experience with other drugs,” Dr. Salgia said.
Factors such as the side effect profile of each drug and insurance coverage could also affect the choice, Dr. Ghafoor said.
Lung cancers with ALK alterations “have unique patterns of metastasis,” Dr. Salgia explained, including to the stomach, peritoneum, and other sites in the body “that other lung cancers don’t tend to metastasize to.”
Moving forward, he continued, studies can hopefully provide more information about issues such as how the respective ALK inhibitors work against tumors that spread to these sites and whether they work better when combined with radiation or other therapies.
But for now, he said, oncologists who regularly treat lung cancer “are just happy that we’re developing these new drugs, that they’re effective, and that the [tumor] responses are deep and durable.”
For Stage III NSCLC, A New Standard of Care?
The durvalumab trial, called PACIFIC, involved a very different group of patients. People in the trial—which was funded by the drug’s manufacturer, AstraZeneca—had stage III NSCLC, which means the cancer has progressed in and around the lungs but has not spread widely in the body.
Although patients with stage III disease can sometimes be treated with surgery, the extent of the disease in patients in PACIFIC was such that surgery was not considered a viable treatment option. In addition, the patients in the trial had to have responded to treatment with the current standard, chemotherapy and radiation—that is, their tumors had already reduced in size following this treatment.
Participants in the trial were randomly assigned to receive durvalumab or a placebo once every 2 weeks for up to 12 months or until their cancer began to progress.
An earlier analysis of the PACIFIC trial showed that patients treated with durvalumab—an immune checkpoint inhibitor that targets the protein PD-L1—lived substantially longer without their disease progressing than patients treated with placebo. The FDA approved durvalumab earlier this year based on those initial results.
The results presented in Toronto come from a longer-term follow-up analysis of the trial. The analysis confirmed the improvement in progression-free survival and showed that patients treated with durvalumab also lived substantially longer overall, reported the study’s lead investigator, Scott Antonia, M.D., Ph.D., of the Moffitt Cancer Center in Florida.
At 2 years after initiating treatment, 66% of patients who received durvalumab were still alive, compared with 55% of patients who received a placebo. More patients treated with durvalumab experienced serious side effects (30.5% versus 26.1%) and more had to stop treatment because of side effects (15.4% versus 9.8%).
A concern going into the trial was lung inflammation, a known and potentially deadly side effect of durvalumab, Dr. Antonia explained during a press briefing. But that concern was not borne out, he noted, with lung inflammation being no more common in patients treated with durvalumab than in those treated with chemotherapy and radiation.
“This is a new standard of care … for patients with this disease,” Dr. Antonia said.
The researchers analyzed survival outcomes in patients according to whether their tumors expressed PD-L1, which has been closely studied as a potential biomarker that can identify patients who are likely to respond to treatment with immune checkpoint inhibitors.
Patients whose tumors didn’t express PD-L1 didn’t seem to benefit from durvalumab, the analysis showed. Dr. Ghafoor cautioned, however, that the PD-L1 analysis was not a planned part of the trial and that the drug can be used to treat all patients, regardless of PD-L1 status.
One group in which some caution in using durvalumab might be warranted is patients whose tumors have mutations in the EGFR gene, said Joshua Bauml, M.D., who specializes in lung cancer at the University of Pennsylvania Abramson Cancer Center and was not involved with the study.
It’s not clear from the trial results whether patients with EGFR mutations who were treated with durvalumab had improved survival, Dr. Bauml noted.
In clinical practice, patients with EGFR mutations whose cancer progresses despite durvalumab treatment might go on to receive an EGFR-targeted therapy, of which several are approved by FDA. But in several small studies, Dr. Bauml noted, use of an EGFR-targeted drug in combination with PD-L1 targeted agent greatly increased the risk of serious side effects in the lungs.
“So we need to talk with our patients with EGFR [mutations] who have completed chemoradiation, walk through these issues, and come to a conclusion about what the best option is for them,” he said.