June 14, 2018, by NCI Staff
Adding the drug nelarabine (Arranon) to standard chemotherapy improves survival for children and young adults newly diagnosed with T-cell acute lymphoblastic leukemia (T-ALL), according to new results from an NCI-sponsored Children’s Oncology Group (COG) clinical trial.
The trial was the largest ever conducted for patients with newly diagnosed T-ALL and T-cell lymphoblastic lymphoma (T-LL).
Four years after starting treatment, among those patients with T-ALL who had a moderate or high risk of their cancer returning, 89% of those who received nelarabine in addition to standard chemotherapy had no signs or symptoms of leukemia, compared with 83% of those who received chemotherapy alone.
“These results are practice changing and identify a new standard treatment for children with T-ALL,” said Malcolm Smith, M.D., Ph.D., of NCI’s Division of Cancer Treatment and Diagnosis, which helps fund COG.
ALL is the most common cancer in children, and T-ALL represents about 10% to 15% of all childhood ALL diagnoses.
An improvement in 4-year disease-free survival is noteworthy because most relapses of T-cell cancers occur in the first 3 or 4 years after diagnosis, said lead author Kimberly Dunsmore, M.D., of Virginia Tech Carilion School of Medicine.
Dr. Dunsmore presented the new findings at a May 16 press briefing ahead of the 2018 American Society of Clinical Oncology annual meeting in Chicago.
Trial Designed to Answer Two Questions
The COG AALL0434 trial, begun in 2007, enrolled 1,895 patients ranging from 1 to 30 years old. Most patients in this phase 3 trial (94% of participants) had T-ALL; the rest had T-LL.
All patients in the trial received standard chemotherapy as well as radiation therapy to the head to prevent or treat cancer that may have spread (metastasize) to the brain or central nervous system.
The trial included four treatment groups, reflecting the fact that it included two separate randomizations to answer two different questions.
First, the trial compared two ways of giving the drug methotrexate (Trexall, Rheumatrex) as part of the interim maintenance phase of chemotherapy treatment. Study participants were randomly assigned to receive either high-dose methotrexate (which requires hospitalization and additional medication to reduce side effects) or escalating-dose methotrexate (which involves starting with a low dose and gradually increasing the dose over time and can be done on an outpatient basis).
Second, the study asked whether adding nelarabine to standard therapy is superior to standard therapy alone in those patients judged to have a moderate or high risk of cancer recurrence. So those patients were also randomly assigned to receive or not receive six 5-day courses of nelarabine, which specifically targets T cells.
As the COG investigators reported previously, the 4-year disease-free survival rate was higher among patients who received escalating doses of methotrexate than among patients who received a very high dose of methotrexate. These findings contrasted with results from previous, smaller trials.
Patients in the trial who received both nelarabine and escalating-dose methotrexate had the best outcomes. This group of patients had a 4-year disease-free survival rate of 91%, which “is spectacular for pediatric T-ALL,” Dr. Smith said.
Patients with T-ALL who failed to go into remission after 29 days were treated with high-dose methotrexate and nelarabine. Those patients had a disease-free survival rate of 55% at 4 years. “To put that into historical perspective, patients in [a large number of] other studies had a disease-free survival rate of 19%,” Dr. Dunsmore noted.
Although nelarabine can have serious side effects, particularly in the nervous system (neurotoxicity), adverse side effects in general were not markedly different between the four treatment groups in the trial. And neurotoxicity, including peripheral and sensory neuropathy, occurred in 6% to 9% of participants in all four treatment groups, Dr. Dunsmore said.
The Food and Drug Administration (FDA) granted accelerated approval to nelarabine in 2005 for the treatment of T-cell leukemia or lymphoma that has not gotten better with other treatments or has recurred after earlier chemotherapy.
That provisional approval was based on results of two earlier NCI-funded phase 2 clinical trials, one in children and one in adults, Dr. Smith said. The COG trial was designed in the hopes of providing evidence to support a full FDA approval for nelarabine in newly diagnosed patients, he explained.
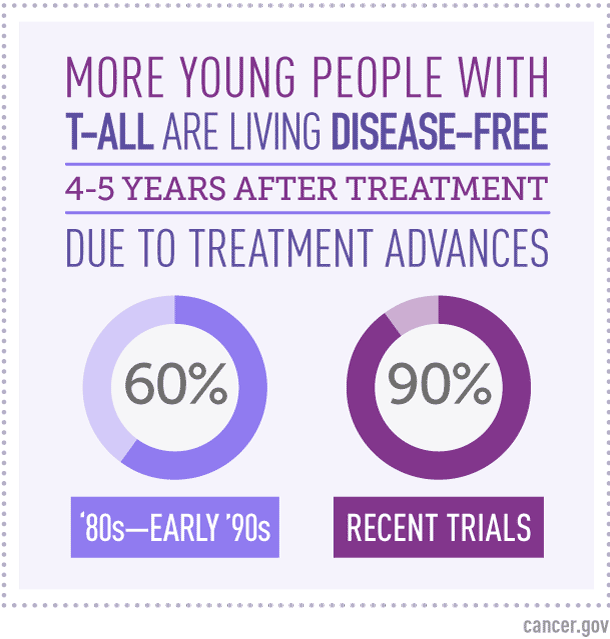
A Dramatic Improvement in Outcomes Since the Early 1990s
As recently as the early 1990s, Dr. Smith noted, 4-year disease-free survival rates for children with T-ALL were only about 60%. To achieve 4-year disease-free survival of more than 90% “is really a remarkable advance,” he said.
Furthermore, 90% of all patients in the trial, regardless of which treatment they received, were still alive after 4 years.
“These are the best outcomes reported to date in a COG trial for children with this cancer, and they are equivalent to or better than outcomes that have been reported by [other clinical trial] groups throughout the world,” Dr. Dunsmore said.
Until recently, the best 4- to 5-year disease-free survival rates for childhood T-ALL hovered around 80%.
Although the patients in the COG trial received lower doses of radiation than were used in the past, most oncologists are moving away from using any radiation to treat ALL, when possible, to avoid the risk of late effects, Dr. Smith said. These effects can include cognitive problems, learning disabilities, and hormonal problems, as well as new cancers that develop in the irradiated area.
“Some clinicians are already using nelarabine similarly to the way it was used in this trial and omitting the radiation,” Dr. Smith said. But, he added, “we’ll need more research before we can ensure that a nelarabine treatment regimen without cranial irradiation is an appropriate standard of care.”
The COG researchers plan to follow patients in the trial for several more years, “as we do for all COG studies, so we can make sure patients are maintaining the results that we’ve seen and to try to understand what future complications may arise,” Dr. Dunsmore said.