, by Norman E. Sharpless, M.D., and Dinah Singer, Ph.D.
It’s hard to believe that 4 years have passed since the Cancer Moonshot℠ was “launched.” In December 2016, Congress passed the 21st Century Cures Act, which authorized $1.8 billion in funding for the Cancer Moonshot over 7 years. Even though it is only at the midway point in terms of funding, we believe it is well on its way to producing meaningful improvements for people with cancer.
Supporters heralded the Cancer Moonshot as “an enormous, once-in-a-lifetime opportunity for the cancer community and our nation to come together around a single disease that touches everyone.”
The architects of the Moonshot articulated three ambitious goals for the Cancer Moonshot: to accelerate scientific discovery in cancer, foster greater collaboration, and improve the sharing of data.
As Congress was completing legislative action, NCI gathered a Blue Ribbon Panel to develop the scientific direction for the Cancer Moonshot and make recommendations for transformative research. Informing the deliberations were nearly 1,600 comments and ideas from stakeholders across the cancer community. Today, we see remarkable progress toward these goals and important changes in the culture of cancer research.
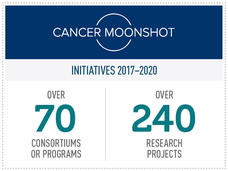
To date, NCI has invested nearly $1 billion in Moonshot funding, supporting over 240 research projects across more than 70 cancer science initiatives. That investment has led to many important insights tied to the Moonshot’s key research priorities, from improving and expanding cancer immunotherapies to finding ways to overcome treatment resistance to identifying new targets for pediatric cancer.
By focusing on areas of cancer research that are most likely to undergo rapid translation to the patient as a result of new investment, the Moonshot has brought together a large community of investigators and clinicians who are dedicated to expediting research to benefit people with cancer and their loved ones.
Improving Immunotherapy
In recent years, drugs that enhance the immune system’s ability to detect and kill tumor cells—known as immunotherapy—have benefited many patients with certain types of cancer, including hard-to-treat cancers like melanoma and lung cancer. The Blue Ribbon Panel made recommendations to expand the benefits of immunotherapy for children and adults with cancer.
One of the recommendations is to establish a cancer immunotherapy research network to develop immune-based approaches for the treatment and prevention of cancer in adult patients. Among the Moonshot-supported groups working on this recommendation is the Immuno-Oncology Translational Network, which has accelerated the discovery of new immune targets for cancer treatments.
Research supported by the Immuno-Oncology Translational Network has led to insights into the responses of ovarian cancers to immunotherapy and into the use of immunotherapy in certain types of head and neck cancers.
Another effort is developing a cancer immunotherapy research network to address challenges in the development of immunotherapies for childhood cancers. The Pediatric Immunotherapy Discovery and Development Network (PI-DDN) has been studying major barriers to the development of immune-based treatments for children and adolescents, such as the lower expression of proteins that can be recognized by immune cells and the immunosuppressive environments of tumors in some childhood cancers.
A recent study supported by this network suggested that some children with B-cell acute lymphoblastic leukemia that recurs or resists treatment may benefit from certain immunotherapy drugs.
Childhood Cancers
Researchers have learned that the major drivers of pediatric cancers are rogue proteins known as fusion oncoproteins. Developing a better understanding of how these proteins function and how they transform normal cells into cancer cells is critical for advancing progress against pediatric cancer.
To implement the Blue Ribbon Panel’s recommendation to intensify research into drivers of childhood cancers, NCI has created the Fusion Oncoproteins in Childhood Cancers (FusOnC2) Consortium.
The consortium brings together researchers with expertise in structural biology, medicinal chemistry, and pharmacology, and includes patient advocates from St. Baldrick’s Foundation and Alex’s Lemonade Stand Foundation for Childhood Cancer. It is specifically focused on building the evidence base in pediatric cancers that are at high risk for treatment failure, or for which there are currently no effective targeted therapies.
Increased attention to this important but understudied field could help overcome existing barriers to progress and pave the way to novel therapeutic approaches with increased efficacy and fewer side effects than current treatment options.
The consortium’s researchers have developed a number of novel cancer models to study these rare cancers and have made significant advances in understanding how the fusion affects the protein’s function and localization in the cell. The FusOnC2 Consortium is highly collaborative, both within the network and with other Moonshot programs, especially the PI-DDN.
Tumor Atlases
Another Moonshot recommendation is to generate human tumor atlases that describe the various cellular, structural, and molecular characteristics of human cancers over time. Toward this end, the Human Tumor Atlas Network (HTAN) is creating detailed spatial and temporal “maps” of a variety of cancers that will be used to investigate how cancer might develop, spread, or respond to treatment.
The network has collaboratively developed best practices for tissue and biospecimen collection and storage, standard methods for data collection and quality control, and policies for sharing data and samples within HTAN and with the broader research community. They have also defined, shared, and integrated best practices that support rigorous, reproducible data analysis and open science.
Six HTAN centers working together across the country are comparing multiplexed imaging modalities and will build data sharing infrastructure, develop image analysis pipelines, and provide guidance to the research community regarding strengths and weaknesses of the various imaging approaches.
Cancer Prevention and Early Detection
The Blue Ribbon Panel recommended investing in research to reduce cancer risk and cancer disparities through the expanded use of proven strategies for cancer prevention and early detection.
The Cancer Center Cessation Initiative was created to address this recommendation, helping cancer centers build and implement sustainable tobacco cessation treatment programs for patients with cancer. More than 50 NCI-Designated Cancer Centers have added smoking cessation programs. These centers refine electronic medical records and clinical workflows to ensure routine delivery of evidence-based tobacco cessation treatment services.
The Abramson Cancer Center of the University of Pennsylvania, for example, has shown that a simple set of decision-support tools provided to the patient, combined with support from the institution, can help increase the number of cancer patients who engage in treatment to help them quit tobacco.
Accelerating Colorectal Cancer Screening and follow-up through Implementation Science (ACCSIS) is another Moonshot-supported effort to expand the use of effective early-detection strategies. ACCSIS is working to build the evidence base on multilevel interventions to increase rates of colorectal cancer screening, follow-up, and referral to care. ACCSIS focuses on underserved groups, including racial and ethnic minority populations and people living in rural or difficult-to-reach areas.
Engaging with Patients
The Moonshot has aimed to modernize how patients are involved in clinical cancer research, which is a critical driver of cancer progress. One approach pioneered by the Moonshot has been to establish patient-centric networks to improve outreach and engagement.
For example, the Participant Engagement and Cancer Genome Sequencing Research Network is using direct participant engagement approaches to promote cancer genome sequencing programs. The network is focused on rare cancers, highly lethal cancers, cancers that occur at an early age, cancers that disproportionately affect certain populations, and cancers that are prevalent in understudied populations.
Another example of Moonshot efforts aimed at improving patient engagement is the NCI Comprehensive Oncology Network Evaluating Rare Central Nervous System Tumors (NCI-CONNECT). This network creates opportunities for patients, health care providers, researchers, and community organizations to work in partnership.
NCI-CONNECT focuses on 12 types of rare tumors of the central nervous system. Patients with these rare tumors can learn more about their cancers, find referrals to experts, participate in studies about risk factors, and learn about ongoing clinical studies through NCI-CONNECT. The network has established partnerships with 9 patient advocacy groups and includes 32 clinical sites across the country that provide access to new clinical trials for these rare tumors.
A related program is MyPART: My Pediatric and Adult Rare Tumors Network. MyPART is working with advocacy groups to raise awareness about rare tumors among researchers, increase access to biospecimens for rare tumor research, and connect patients with investigators. The network has already developed a pipeline for biospecimen collection and analysis, a strong communication platform with patients and patient advocates, and established several new specialized rare tumor clinics around the country.
Greater Collaboration
As these examples illustrate, enhanced collaboration among investigators—and patients and advocates—can be a valuable accelerant of research progress. To foster greater collaboration, many Moonshot programs work as interconnected networks, designed to leverage the strengths and expertise of all investigators and partners involved. An explicit goal is to break down organizational and academic silos that can develop at research institutions and slow progress.
Each Moonshot-supported research network includes multiple groups of investigators focused on accelerating progress in certain areas of cancer research. Researchers work closely with others within their network, often communicating regularly and sharing data and resources, as well as with members of other networks. NCI has also convened meetings to gather multiple Moonshot networks, leading to still more collaboration and innovation.
Dinah Singer, Ph.D.
NCI Deputy Director for Scientific Strategy and Development
Sharing information within Moonshot research networks and with the broader cancer research community has been critical to the initiative’s progress and success to date. Within the research networks, sharing data and research tools is allowing investigators to tackle challenges that are beyond the scope of individual groups.
Enhanced Data Sharing
The Moonshot embraced an approach of “radical data sharing.” Through the Moonshot data-sharing policy, new findings and underlying data from all Moonshot-funded studies are made publicly available as promptly as possible.
To facilitate dissemination of new findings, all publications of Moonshot-supported research must be published in open-access format, available to all, free of charge. This approach has been so successful that it has become a model for other research projects across NCI and the National Institutes of Health.
NCI leadership worked with top-tier, peer-reviewed journals to provide an open-access option for Moonshot papers, even for journals that did not previously have a mechanism to support open access. Unfettered access to these research findings stands to yield benefits in cancer research beyond the Cancer Moonshot, and the precedent established may accelerate progress in other scientific domains as well.
The Blue Ribbon Panel also recommended developing a National Cancer Data Ecosystem to enable and encourage all participants across the cancer research and care continuum to share, access, combine, and analyze diverse data. A number of Moonshot-supported efforts aimed at fulfilling this goal are underway.
The Cancer Research Data Commons (CRDC) is a cloud-based, data science infrastructure that connects data sets with analytics tools to allow users to share, integrate, analyze, and visualize cancer research data to drive scientific discovery. The CRDC currently provides access to 23 datasets, with more to come, and users can bring their own data to combine with the existing data to perform novel analyses through NCI’s cloud resources.
These data science endeavors are complex and resource intensive and their value can be transformative. Across the cancer research community, there is clearly interest in Moonshot data: In 2020 alone, 70,000 users of the cloud-based, Moonshot-supported data infrastructure performed nearly 70 million hours of cancer research analysis.
The Future
As these examples illustrate, the Cancer Moonshot’s strategies and investments are indeed moving cancer research forward. Successful programs are in place to deliver important advances by 2023, the final year of dedicated Moonshot funding.
To maintain the pace of progress established since the Moonshot’s inception, we need to ensure a smooth transition of these programs into traditional research funding mechanisms, without disrupting the important work NCI supports throughout its portfolio at research institutions across the country.
Our vision of the Moonshot’s future draws heavily on the lessons we have learned to date, including the rapid research advances that can come from bringing the community together behind a common goal. The focus going forward will remain on building collaborations, sharing data, and stimulating investigator-initiated research to pursue research questions that are most likely to benefit from new investments.
Half a century after passage of the National Cancer Act of 1971, we are grateful for the passion and creativity of the researchers, physicians, patients, and advocates who provided a foundation for today’s cancer research enterprise. With new ideas and technologies, we are poised to reduce the burden of cancer for people everywhere.