, by NCI Staff
People with advanced multiple myeloma, the second most common blood cancer, may soon have another treatment option, new clinical trial results suggest.
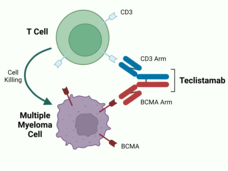
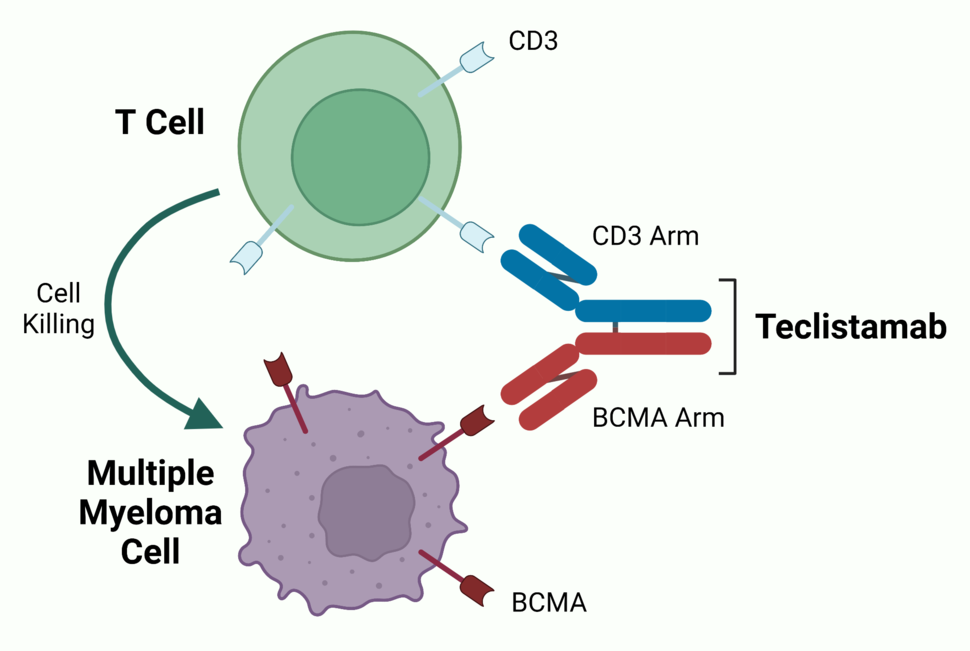
The trial tested an experimental immunotherapy drug teclistamab (Tecvayli) in people with multiple myeloma that did not respond to or came back after at least three different cancer treatments.
In the trial, nearly two-thirds of participants had at least a partial response to teclistamab, and almost 40% had a complete remission of their cancer. In addition, the study found, the median time that patients receiving teclistamab lived without their cancer getting worse was about 11 months. The responses lasted a median of 18 months.
These results make teclistamab “very exciting” compared with other commonly used options for patients who’ve already had a lot of different treatments, said Natalie Callander, M.D., a myeloma expert at the University of Wisconsin Carbone Cancer Center, who was not involved with the trial.
Such patients have few treatment options and usually only live for 8 or 9 months, said Saad Usmani, M.D., chief of the myeloma service at Memorial Sloan Kettering Cancer Center and one of the senior investigators on the trial.
The responses seen with teclistamab are “impressive, and compare favorably to the effects of CAR T-cell therapy,” two of which have been approved for advanced multiple myeloma, said James Kochenderfer, M.D., a senior investigator in NCI’s Center for Cancer Research, who also was not involved with the trial.
In addition, several experts noted logistical challenges of using CAR T-cell therapy, including high cost, limited availability, and the time needed to manufacture the treatment.
Results of the trial, known as MajesTEC-1, were published August 11 in the New England Journal of Medicine. The trial was funded by Janssen, the maker of teclistamab.
Based on these results, teclistamab was recently approved for use in the European Union to treat adults with multiple myeloma that did not respond to (was refractory) or came back (relapsed) after at least three other treatments for their disease.
The Food and Drug Administration (FDA) has not yet approved the drug for use in the United States, but experts believe it is just a matter of time. Janssen submitted an application to FDA last December to approve teclistamab for this same use in the United States.
If FDA approval comes through, Dr. Callander said, “having this drug available is going to be an important new addition for patients.”
New treatments target BCMA protein on myeloma cells
In multiple myeloma, abnormal plasma cells, a type of white blood cell, form tumors in the bones and other parts of the body. In almost all patients, the cancer eventually comes back, or relapses, after treatment. With each new treatment, the cancer becomes less likely to respond and the time before the disease comes back gets shorter.
Several newer treatments for multiple myeloma—including two recently approved CAR T-cell therapies (one in 2021 and one in 2022), teclistamab, and more than half a dozen similar drugs still in development—target a protein called BCMA. This protein is commonly found on plasma cells but is often found at higher levels on multiple myeloma cells.
Teclistamab is known as a bispecific antibody, meaning it can bind to two different targets at the same time—in this case, BCMA and a protein called CD3, which is present on immune cells called T cells. By bringing myeloma cells and T cells together, the drug helps the T cell to recognize and destroy the tumor cell, Dr. Usmani explained.
Strong treatment responses, concerns about infection risk
The MajesTEC-1 trial enrolled 165 adults with relapsed or refractory multiple myeloma who had previously been treated with at least three types of drugs commonly used to treat multiple myeloma. Half of the study participants had received five previous lines of therapy, and 82% had a previous stem cell transplant.
Participants received teclistamab once a week as an injection under the skin and were followed for a median of 14.1 months. These treatments stopped the cancer from progressing for a median of 11.3 months, Dr. Usmani and his colleagues found. The team is continuing to follow patients to see how long they live while on teclistamab.
Because BCMA is found on normal plasma cells as well as on myeloma cells, treatments that target BCMA can also reduce the production of infection-fighting antibodies. Indeed, in the trial, the most common serious side effects were infections, along with low white and red blood cell counts. Almost all participants (95%) had at least one serious side effect.
Of note, 12 of the 165 participants in the trial, which began enrolling patients in March 2020, died of COVID-19. Doctors will need to be aware of the increased risk of infections in patients who are receiving teclistamab and take steps to reduce that risk when possible, Dr. Callander said.
The most common side effect seen with teclistamab was cytokine release syndrome, a potentially serious immune reaction that also occurs with CAR T-cell therapy. Although this reaction was mild or moderate in most of the 119 participants who experienced it, nearly all required supportive care to manage the symptoms.
In an attempt to reduce the risk of more severe reactions, participants were given two smaller “step-up” doses of teclistamab 2–4 days apart before starting on the full dose. Additionally, patients had to stay in the hospital when receiving the step-up doses and first full dose, because cytokine release syndrome typically occurs after the first two or three doses of a bispecific antibody drug.
Pros and cons of CAR T-cell therapy versus bispecific antibodies
In the clinical trials that led to FDA approval of two BCMA-targeted CAR T-cell therapies for advanced multiple myeloma, ide-cel (Abecma) and cilta-cel (Carvykti), response rates were somewhat higher than those seen for teclistamab, ranging from 73% to 98%. The cancer did not progress for about 9 months or longer.
Although they have not been compared head-to-head, teclistamab and CAR T-cell therapy each have advantages and disadvantages, Dr. Usmani said.
Whereas CAR T-cell therapy, which uses a patient’s own T cells to treat their disease, takes at least 5 or 6 weeks to produce “even under the best of circumstances,” teclistamab can be started right away, he said.
In addition, he continued, “with bispecific antibodies you’ll be able to treat older patients who are not eligible for CAR T-cell therapies” based on current FDA criteria.
However, “teclistamab requires weekly infusions [to keep the cancer in check]. In contrast, CAR T cells only require a one-time treatment,” allowing patients to be off all drugs and free of side effects as long as the cancer remains at bay, Dr. Kochenderfer said.
High cost is also a concern with CAR T-cell therapy. And although the cost of teclistamab is not yet known, it is also expected to be high, Dr. Callander said. At least eight or nine other bispecific antibodies for treating multiple myeloma are in the pipeline, including several that also target BCMA, she said, but she does not expect that having more drugs will lead to lower costs.
CAR T-cell therapy requires that patients have access to specialized care centers. It remains to be seen, Dr. Callander said, whether giving teclistamab will require any specialized training or access to specialized facilities, which could limit access to the drug for people being treated in smaller community oncology practices.
Ongoing studies are investigating the use of teclistamab in combination with other drugs for people with multiple myeloma who haven’t already received multiple treatments, Dr. Usmani said. It will also be important to figure out how best (including in what order) to use teclistamab with other treatments that target BCMA, including CAR T-cell therapy, experts said.
Finally, Dr. Kochendorfer noted, “Neither CAR T cells nor teclistamab are likely to cure more than a very small percentage of [people with] multiple myeloma, so research on better treatments is needed.”