, by NCI Staff
Adults with the most common form of acute lymphoblastic leukemia (ALL), a type of blood cancer, often respond to initial treatments only to have the disease return, while others’ cancers don’t respond at all to treatment. Now, these patients may have another treatment option, a type of immunotherapy called CAR T-cell therapy.
On October 1, the Food and Drug Administration (FDA) approved the use of the CAR T-cell therapy brexucabtagene autoleucel (Tecartus) for adults with B-cell precursor ALL that has not responded to treatment (refractory) or has returned after treatment (relapsed). The approval makes brexucabtagene the first CAR T-cell therapy approved for adults with ALL.
The approval was based on results of a small phase 1/2 clinical trial called ZUMA-3 that included more than 50 adults with relapsed or refractory B-cell precursor ALL who were treated with brexucabtagene. Within 3 months of receiving the treatment, more than half of the participants had no signs of cancer (complete remission). And for many of the patients, that response lasted for more than a year.
“We haven’t seen responses and durations of responses like this,” said the trial’s lead investigator, Bijal Shah, M.D., M.S., of Moffit Cancer Center.
This is the second FDA approval for brexucabtagene. In August 2020, it was approved to treat some adults with mantle cell lymphoma.
Consistent with previous studies of brexucabtagene, most participants experienced side effects, including high rates of a serious inflammatory condition called cytokine release syndrome, or CRS, and neurological problems. Two participants died as a result of an adverse reaction to the treatment.
While the side effects are often severe, they don’t outweigh the potential benefits of brexucabtagene, Dr. Shah said. “We are going to see these syndromes, but for [most] patients, they are going to be reversible. They’re going to get better, and they have potential remission on the other side.”
A New Approach to Treating a Stubborn Leukemia
As its name indicates, B-cell ALL originates in white blood cells called B cells. It occurs most commonly in children but can also affect adults.
Over the past 2 decades, researchers have been able to greatly improve how long children with B-cell ALL live, in large part by modifying and improving the chemotherapy regimens they receive.
Using some of those same regimens, “we’ve seen outcomes improve for adolescents and young adults up to age 39 with B-cell ALL,” said Shira Dinner, M.D., of Northwestern University’s Feinberg School of Medicine, who was not involved in the ZUMA-3 trial.
But similar gains haven’t been seen for older people with B-cell ALL, she added.
“For adults 40 and older, the prognosis has still been poor.”
While adults with B-cell ALL may respond to other drugs, including blinatumomab (Blincyto) and inotuzumab ozogamicin (Besponsa), the cancer often returns. The median overall survival after treatment with either drug is less than 8 months.
Even though outcomes have improved with these new treatment options, Dr. Dinner noted that “some of the limitations of those treatments, as well as of more traditional chemotherapies, is that the duration of remission is still very short.”
When the cancer does return after treatment with these drugs, the next step is often a stem cell transplant. But this procedure is not an option for many patients. Some “have already had a transplant, or don’t have a donor available, or are just not fit to have a transplant due to their age or other medical problems,” said Dr. Dinner.
“For patients who don’t have the option of a stem cell transplant, [brexucabtagene] could be a good alternative,” Dr. Dinner continued.
Brexucabtagene was associated with promising survival outcomes. More than half of patients in the ZUMA-3 trial whose tumors responded to the treatment were still alive 22 months later, with median survival not reached.
“I can’t say yet [that brexucabtagene] is a replacement for a stem cell transplant, but … for duration of response and survival outcomes to date, it is the most promising of the treatments that we have available,” Dr. Dinner noted.
Results from the ZUMA-3 Trial
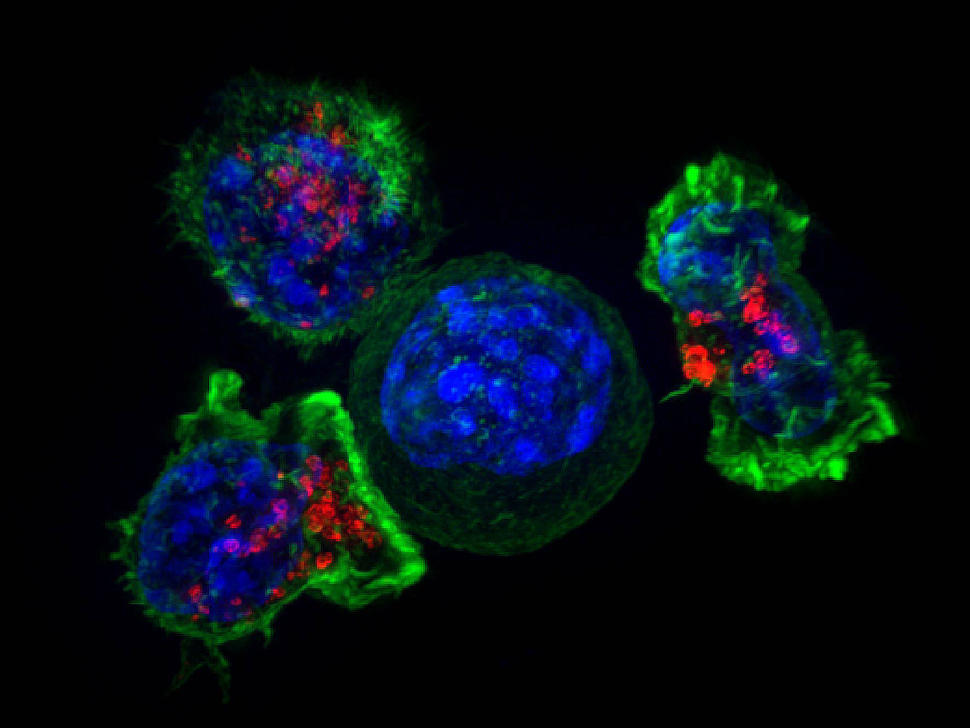
Like other FDA-approved CAR T-cell therapies, brexucabtagene begins with a patient’s own T cells. These immune cells are collected and then modified to produce a special receptor on their surface through a complex manufacturing process. This receptor allows the T cells to better target and kill cancer cells.
Of the patients who initially enrolled in the trial, CAR T cells were successfully manufactured for all but 6. The median time between T cell collection (via a procedure known as leukapheresis) and the production of CAR T cells was 13 days for patients in the United States and 14.5 days for patients in Europe.
Before being infused with brexucabtagene, all participants underwent conditioning chemotherapy.
According to Kite, which manufactures brexucabtagene and funded the ZUMA-3 trial, at a median follow-up of approximately 12 months, 65% of patients had a complete remission following treatment or a complete remission without their white blood cell counts returning to normal. Of those who had a complete remission, Kite reported, more than half continued to have no signs of cancer for at least a year.
This does not necessarily mean that these patients are completely cured, Dr. Dinner cautioned. “We will need longer-term follow-up data on these patients” to determine whether the cancer could return, she noted.
Dr. Shah is optimistic. Following brexucabtagene treatment and a stem cell transplant, he said, “there’s a real chance that you could carry that patient forward to a cure. And for those who can’t [undergo a transplant], they’re still really good odds—certainly higher than we’ve ever achieved in this setting.”
Nearly every patient experienced side effects after receiving brexucabtagene. CRS, which can cause intense fever and other flu-like symptoms, occurred in 92% of people treated with brexucabtagene, with 26% of patients experiencing severe CRS. And 87% of participants experienced neurological side effects, including tremor, confusional state, and encephalopathy. For 35% of patients, the neurological side effects were severe.
“When these symptoms do happen, it could be very hard for an older adult to tolerate [them],” Dr. Dinner said. “So I do think [side effects are] an important factor that a physician needs to be considering when discussing the risks and benefits [of brexucabtagene] with a patient.”
What Lies Ahead for Brexucabtagene
Although the results from this trial are promising, said Dr. Dinner, more research is needed to understand how the therapy will fit into existing treatment strategies.
“We have to be mindful of the fact that [CAR T-cell therapy] is a complicated new technology,” Dr. Dinner said. For example, hospitals and clinicians are still learning how to deal with the “logistical hurdles” presented by these treatments.
Nonetheless, she added, “this is a big step forward.”
Based on these encouraging results in people with refractory or relapsed B-cell ALL, CAR T-cell therapy could be investigated as an initial, or first-line, treatment for some patients, according to Dr. Shah. That could include those whose cancer is determined to be at particularly high risk of not responding well to existing first-line treatments.
There are also potential uses for brexucabtagene or other CAR T-cell therapies in other patients with ALL. “This [approval] is only for B-cell precursor ALL,” Dr. Dinner said. “So there are still gaps for T-cell ALL, which makes up 25% of the ALL population. There are some CAR T-cell treatments that are in development for that disease.”