Results from a recent study from China showed that a new approach to chimeric antigen receptor (CAR) T-cell therapy was able to put B-cell acute lymphoblastic leukemia (ALL) into remission in many children with high-risk disease. In an article published in the Journal of Clinical Oncology, the study authors reported that 99% of the 194 patients in the clinical trial had undetectable disease after the treatment.
ALL is the most common type of childhood cancer. For every 4 people under age 20 diagnosed with leukemia, 3 of them are diagnosed with ALL. In this study, the participants had ALL that was resistant to treatment or whose cancer had returned more than once after treatment.
In CAR T-cell therapy, some T cells are removed from a patient’s blood and are changed so they have specific proteins called receptors. The receptors allow the changed T cells to recognize the cancer cells. When the changed T cells are returned to the patient’s body, they seek out and destroy cancer cells.
The researchers in this study were able to develop the CAR T cells in an average of 7 days. Most methods for developing CAR T cells take about 1 month. The CAR T cells in this clinical trial were also designed to have 2 target antigens: CD19 and CD22. Other CAR T cells usually only target 1 antigen.
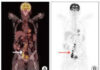
There were 194 Chinese participants in this phase II study with ALL that had not responded to treatment or returned after treatment. When the treatment began, 70% of the participants were younger than 10 years old. Across all 194 patients with high-risk ALL, 192 (99%) had a complete remission after the treatment. At 12 months, nearly 74% had no signs or symptoms of the disease. This is called event-free survival. But in 43 patients, the leukemia returned.
There were 78 patients who had a bone marrow/stem cell transplant after receiving CAR T-cell therapy to destroy any remaining cancer cells. Among these patients, the 12-month event-free survival was 85%, compared to about 69% among the 116 participants who did not receive a stem cell transplant. There were 20 patients who had the disease return in the testicles, and the 12-month event-free survival for them was 95%. Another 10 patients had the disease return in the central nervous system, and their 12-month event-free survival was nearly 69%.
Most (88%) of the participants in this study experienced cytokine release syndrome, a potential side effect of immunotherapy in which the immune system goes into overdrive. About 21% of the patients developed nervous system side effects. Three participants died from side effects of the treatment.
“We believe that this regimen may become the new standard of care, in part because it spares some patients from the use of local radiation to the bone marrow, the testes, or the central nervous system, which can cause life-altering side effects, such as sterilization, severe neuro-cognitive impairment, and brain tumors. To put this study in context, the first child who received CAR T-cell therapy for B-ALL after multiple relapses has recently celebrated her 10-year cancer-free survival milestone, and we hope that our finding will result in many more such milestones.”
—corresponding author Ching-Hon Pui, MD
St. Jude Children’s Research Hospital
Memphis, Tennessee