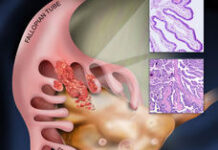
The researchers from the The University of Texas MD Anderson Cancer Center evaluated HER2 expression by immunohistochemistry (IHC) in 4701 patients with different solid tumours. HER2-low expression, defined as 1-2+ by IHC was common across many solid tumours (41.1%). Furthermore, a significant number of patients without HER2 alterations (35.7%) had HER2 expression (≥1+).
The findings suggest that many patients with HER2-low solid tumours might benefit from HER2-targeted therapies. The study team led by Ecaterina Dumbrava, Assistant Professor in the Department of Investigational Cancer Therapeutics published the findings on 22 August 2023 in the Annals of Oncology.
HER2 overexpression or amplification is a validated prognostic and predictive biomarker in breast and gastric/gastro-oesophageal (GEJ) cancers. Several HER2-targeted agents have been approved and improved outcomes for patients with HER2-driven malignancies. Recently, trastuzumab deruxtecan was approved for the treatment of HER2-low (defined as IHC 1+ or IHC 2+/in situ hybridisation [ISH]-negative) breast cancer and for non-small cell lung cancer (NSCLC) with HER2 mutations.
The authors wrote in the background that HER2 status classification has been evolving and while there are clear ASCO/CAP guidelines for breast and gastric cancers, other tumour types have no clear guidelines for testing. Classically, HER2 positivity is defined as overexpression by IHC or HER2 gene amplification by ISH. Most recently HER2-low is a newly defined category with HER 1+ or 2+ expression by IHC and lack of HER2 gene amplification measured by ISH. These patients have been previously considered HER2-negative.
The frequency of HER2-low expression is unknown across solid tumours. Furthermore, the changes in HER2-low expression status between primary and metastatic samples are unknown. The association between the HER2 genomic alterations and HER2 expression also remains unknown. To address these gaps, the authors studied a large cohort of patients with solid tumours and evaluated their HER2 expression. They also sought to assess changes in HER2 status between paired primary and metastatic breast and gastric/GEJ cancer samples.
HER2 expression was evaluated by IHC in 4701 patients with solid tumours. The study team evaluated the HER2 expression by IHC and amplification by ISH in paired breast and gastric/GEJ primary and metastatic samples. HER2 expression was correlated with HER2 genomic alterations evaluated by next-generation sequencing (NGS) in non-breast, non-gastric/GEJ samples.
HER2 expression (HER2 IHC 1-3+) was found in half (49.8%) of cancers, with HER2-low (ICH 1 or 2+) found in many tumour types: 47.1% in breast, 34.6% in gastric/GEJ, 50.0% in salivary gland, 46.9% in lung, 46.5% in endometrial, 46% in urothelial and 45.5 % of gallbladder cancers.
The concordance evaluation of HER2 expression between primary and metastatic breast cancer samples, showed that HER2 3+ remained unchanged in 87.1% with a strong agreement between primary and metastatic samples, with a weighted kappa of 0.85 (95% confidence interval 0.79-0.91).
HER2 alterations were identified in 117 patients (7.5%) with non-breast, non-gastric/GEJ solid tumours who had NGS testing. Of 1436 patients without HER2 alterations, 512 (35.7%) showed any level of HER2 expression by IHC.
The authors commented that a more tumour agnostic analysis of HER2 expression is warranted given the recent FDA approval of HER2-targeted treatments for HER2-low breast cancer and NSCLC with HER2 mutations and the inclusion in NCCN guidelines of HER2-targeted therapies for non-breast and non-gastric/GEJ tumour types including colon, salivary gland, and biliary truct cancers.
This is the first study to report on HER2-low expression in a large cohort of patients across multiple solid tumour types who underwent HER2 testing for personalised cancer therapy. Changes in HER2 status between paired primary and metastatic breast and gastric/GEJ samples provide insight into the potential role for re-testing of HER2 expression. Further prospective research is needed to evaluate the clinical outcomes by tumour types and HER2 expression status.
This work was supported by the Strategic Alliance: Sanofi−MD Anderson Cancer Center HER2 T-Cell Engager Project Collaboration, MD Anderson Cancer Center Support grant from US NIH/NCI, Center for Clinical and Translational Science award.