, by NCI Staff
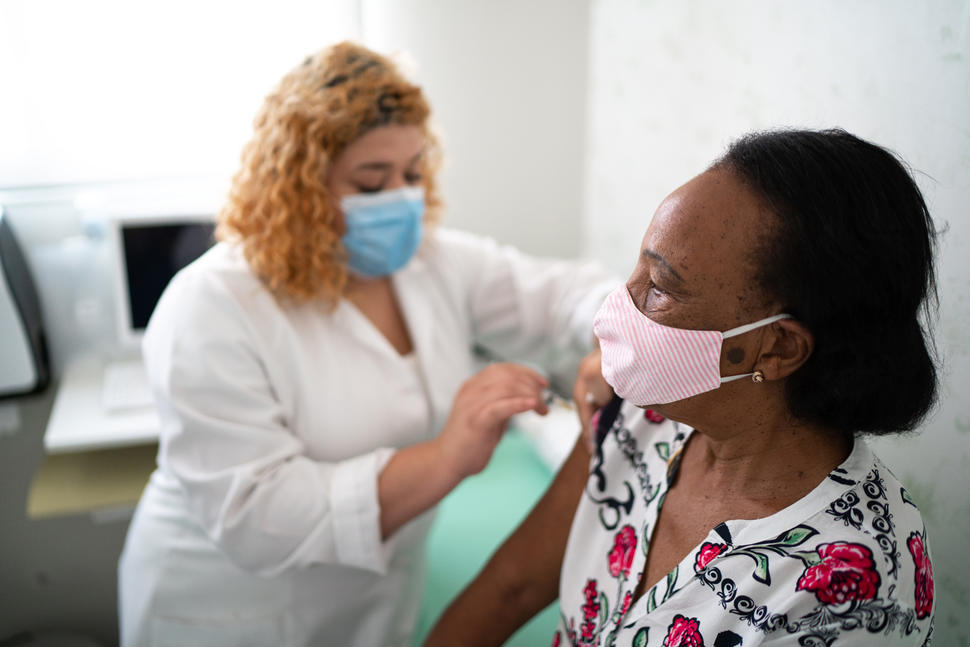
Doctors have generally recommended that their patients with cancer receive vaccines to protect against infection with SARS-CoV-2, the virus that causes COVID-19. But some people with cancer may not be as protected by the vaccine as people without the disease, results from three new studies suggest.
The findings provide some of the first data on the efficacy of COVID-19 vaccines in people with cancer, who were largely excluded from the initial trials testing the vaccines. Three groups working independently in the United States, the United Kingdom, and France conducted the studies.
Two of the studies found that COVID-19 vaccines might not stimulate effective immune responses in some people with blood cancers. These findings highlight the need for more research on this group in particular, the investigators said.
“Patients with blood-related cancers often have dysfunctional immune systems, and as a result they’re just not able to respond as well to the COVID-19 vaccine as other people,” said Elad Sharon, M.D., M.P.H., a senior investigator at NCI, who was not involved in the new studies but is leading a clinical trial testing COVID-19 vaccines in people being treated for cancer.
The new findings, Dr. Sharon added, are consistent with previous studies showing that people whose immune systems may have been weakened by cancer or its treatments may not develop effective immune responses to the flu vaccine.
Profiling Vaccine Immune Responses in Patients with Blood Cancers
In the US study, nearly half of the patients with blood cancers—31 out of 67 patients (46%)—did not produce detectable antibodies to the SARS-CoV-2 spike protein following two doses of the Pfizer-BioNTech COVID-19 vaccine. The researchers concluded that the 31 patients were “nonresponders” to the vaccine.
“The findings confirm what we have suspected all along, which is that immunocompromised people aren’t going to have the same immune responses to COVID-19 vaccines as people in the initial clinical trials testing these vaccines,” said study leader Ghady Haidar, M.D., of the University of Pittsburgh School of Medicine.
Patients in the study had B-cell chronic lymphocytic leukemia, lymphomas, multiple myeloma, and other blood cancers. Those with B-cell chronic lymphocytic leukemia were the least likely to respond to the vaccine, the researchers found.
Their results appeared on April 7 in medRxiv, a preprint publication. Preprints are complete and public drafts of scientific studies that have not yet been peer reviewed.
The study was small and needs to be confirmed by larger studies, Dr. Haidar cautioned. Another limitation was that the researchers did not determine whether antibodies from vaccine responders were able to neutralize SARS-CoV-2.
Nonetheless, all three new studies provide important information for patients, said Dr. Haidar.
“People with weakened immune systems need to be aware of these results, so they can live their lives safely and reduce the risk of developing COVID-19,” he said. “We don’t want these people to assume that they’re protected when they may very well not be.”
His team is also studying COVID-19 vaccine responses in people with HIV/AIDS, autoimmune conditions, and transplant recipients. These studies, Dr. Haidar noted, should eventually help inform responses to a critical question: What can doctors do for people who do not mount an immune response to the vaccine?
“It’s frustrating to be a physician and not have an answer to this question,” he continued. “But people should not despair. For the time being, they should continue to use masks and social distance until the science catches up and we have something more concrete to offer.”
European Studies Provide Additional Data on Vaccine Responses
The leader of the UK study, Sheeba Irshad, M.D., Ph.D., of King’s College London, echoed these recommendations. “Until more studies looking specifically at COVID-19 vaccines in patients with cancer are available, it is important for patients with cancer to continue to observe all public health measures in place, even after vaccination,” Dr. Irshad said.
Getting vaccinated is also important for those in close contact with people with cancer, she stressed, both to protect the patient and to promote herd immunity more broadly.
In their study, Dr. Irshad and her colleagues analyzed immune responses to the Pfizer-BioNTech COVID-19 vaccine—including antibody production, virus-neutralizing ability, and T-cell responses—in people with and without cancer. After one dose of vaccine, those with cancer generally had weaker immune responses than people without the disease, the researchers reported in Lancet Oncology on April 27.
Researchers assess a person’s response to COVID-19 vaccination in part by measuring the levels of specific antibodies in blood.
Credit: Adapted from the Centers for Disease Control and Prevention
“The findings imply that vaccination with a single dose of the [Pfizer-BioNTech] vaccine leaves most patients with cancer wholly or partially immunologically unprotected,” said Dr. Irshad. The study included 151 people with cancer (95 patients with solid cancers and 56 patients with blood cancers) and 54 people without cancer (known as a control group).
The “extremely poor immune responsiveness” in patients with blood cancers is of particular concern, Dr. Irshad noted, because immunocompromised patients may harbor persistent SARS-CoV-2 infections, potentially leading to the emergence of new variants of the virus.
Within two weeks of a second vaccine dose, immune responses improved substantially among most patients with solid cancers (e.g., breast, colorectal), the researchers found. The study was not large enough to reach conclusions about the effect of a second dose in patients with blood cancers.
The third study, conducted by French researchers, also found differences in immune responses between people with and without cancer. After the first dose of the Pfizer-BioNTech vaccine, nearly half of the 110 patients with cancer showed no antibodies to the SARS-CoV-2 spike protein, they reported April 28 in Annals of Oncology.
The seroconversion rate was only 55% in patients with cancer, though it reached 100% in the 25 people in the control group. Seroconversion refers to the time from vaccination to when antibodies of the virus become present in the blood.
NCI-Supported Trial Will Study Moderna Vaccine
Since these studies appeared, Dr. Sharon and his colleagues have launched an NCI-supported clinical trial at the NIH Clinical Center in Bethesda, MD. The study will assess the ability of the Moderna COVID-19 vaccine to stimulate an immune response in 120 adults who are undergoing treatment for various types of cancer.
Half of the patients will be receiving immunotherapy drugs known as PD-1/PD-L1 inhibitors for solid tumors as part of their care. The others will be undergoing treatment for blood cancers such as leukemia, lymphoma, and multiple myeloma, or will have undergone a stem cell transplant for their cancers.
The research community does not know what effect, if any, treatment with an immunotherapy drug could have on the use of COVID-19 vaccines. Immune checkpoint inhibitors—such as PD-1/PD-L1 inhibitors—enhance the ability of the immune system to detect and attack cancer cells.
“Will the patient get more antibodies as a result of having a PD-1 or PD-L1 on board?” Dr. Sharon asked. “This is the kind of question we’re trying to answer. And depending on what we learn, the results might help us guide further efforts in developing treatments for cancer going forward.”
The researchers will assess immune responses by looking at antibody levels and the activation of T cells associated with SARS-CoV-2 infection in blood and saliva samples. Patients will be tested at planned intervals following the second dose of vaccine—after 1 week, 1 month, 6 months, and a year.
“We actually have the opportunity to explore something—a virus—that no human had ever really had immunity to,” Dr. Sharon said. “This could help us to better understand defects in the immune system and whether there are ways that we can shore up those defenses.”