Cancer Research UK’s spin-out, GammaDelta Therapeutics (‘GammaDelta’), has been given approval from the US Food and Drug Administration (FDA) to trial its unique T-cell therapy in humans.
The trial is expected to begin later this year in the United States and will test the safety and early indications of efficacy of the cell therapy in people with a type of blood cancer called acute myeloid leukaemia (AML).
I’m thrilled to see our research finally reaching patients and look forward to seeing how clinical trials progress.
– Adrian Hayday, co-founder of GammaDelta Therapeutics and ead of the Crick’s Immunosurveillance Laboratory
Unique T cells
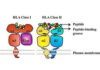
Like many immunotherapy drugs for cancer, GammaDelta’s therapy harnesses the power of a type of immune cell called T cells in an attempt to kill cancer cells.
But in the case of GammaDelta’s therapy, the type of T cells it works with are rather unique.
Known as gamma delta T cells, these cells make up a small number of all the T cells in the body. And while they’re less abundant than other T cells, they have distinct properties that give them huge promise as a potential cancer therapy.
Unlike the more abundant alpha beta T cells, which rely on a specific molecule to help them recognise red flags on cells, gamma delta T cells scan the surface of cells directly to spot potential threats. Because of this, GammaDelta’s T cell therapy could help treat patients whose cancer cells have tried to evade the immune system by removing certain molecules from their surface, making them invisible to alpha beta T cells.
Furthermore, GammaDelta’s therapy is designed to be ‘off the shelf’, meaning the T cells used in the therapy are taken from healthy donors, rather than the patient themself. These ‘off the shelf’ therapies could be more readily available to patients in the future.
Following approval from the FDA, the unique properties of gamma delta T cells will be evaluated for the first time in patients with AML, in a trial expected to begin later in 2021. The hope is this new type of immunotherapy might be able to improve patient outcomes in blood cancers.
A pioneering spin-out company
Gamma Delta Therapeutics, a UK-based biotech, was initially formed in 2016 by Cancer Research UK’s Commercial Partnerships team along with support from the life sciences investor, Abingworth.
It was founded on the pioneering research into these unique gamma delta T cells which was part funded by Cancer Research UK and led by Adrian Hayday and Oliver Nussbaumer of the Crick and King’s College London.
Hayday said he was “thrilled” to see the research finally reaching patients. “From our very first studies into γδ T cells in the 1980s, through to demonstrating their unique activity in the presence of cancer, it’s been clear that these immune cells have enormous potential for the development of new immunotherapy treatments.”
Cancer Research UK’s Commercial Partnerships team develops promising ideas into successful cancer therapeutics, vaccines, diagnostics and enabling technologies. The team’s deep understanding of both academia and industry enables them to translate research into commercial propositions to deliver patient benefit and commercial value that will support further cancer research. Creating spin-out companies is one of the ways Cancer Research UK gets early stage technology out of the research lab and on to the path to being developed towards a product.
GammaDelta is one of many spin-out companies whose conception has been supported by Cancer Research UK.
The charity has an established track-record with spin-outs, having been involved in the creation of, or supporting our university partners to create, over 43 spin out companies which have secured over £2.3 billion in investment to date.