, by NCI Staff
Scientists may have found an important clue as to why immunotherapies—treatments that stimulate the immune system to fight off cancer—tend to work for people with lung cancer but not for those with pancreatic cancer. In part, the answer may lie in the number of special immune cells, called dendritic cells, in the two types of tumors.
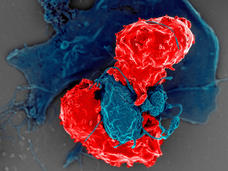
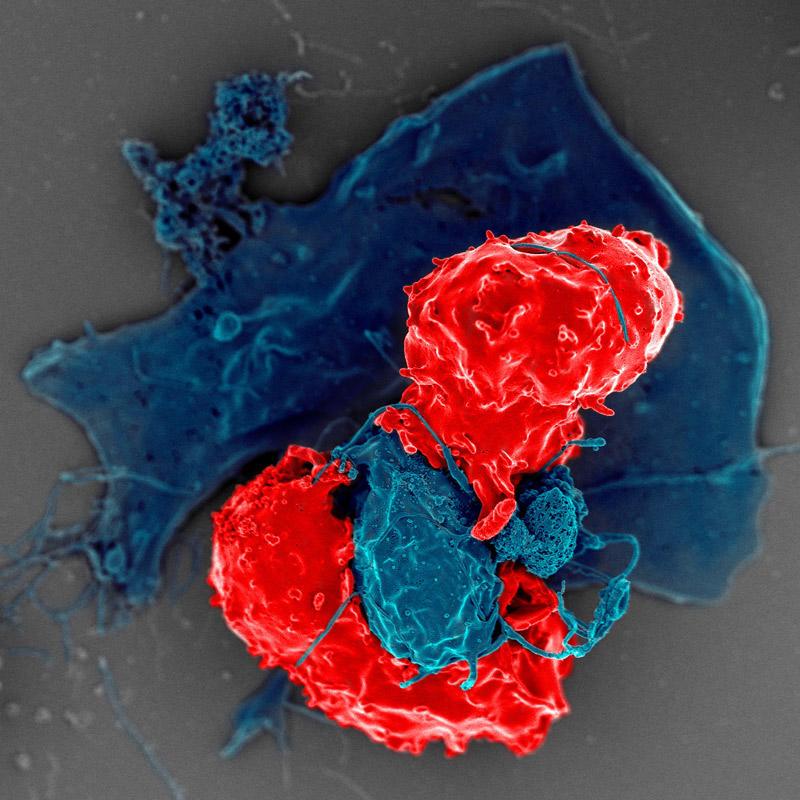
Dendritic cells are part of the immune system’s first line of defense against cancer and infections. They patrol the body, searching for abnormal or infected cells. If they find one, they eat the offender and show pieces of it to an army of immune cells, like holding up a “wanted” poster. The army then hunts down and attacks the cancer or infection.
In mouse models, pancreatic tumors had far fewer and less active dendritic cells than lung tumors, the scientists found. Without dendritic cells, other immune cells in the pancreatic tumors didn’t recognize cancer cells as a threat, they found.
But treating the mice with drugs that boost the number and activity of dendritic cells triggered an immune response that slowed the growth of pancreatic tumors, the researchers discovered. Combining the drug treatment with radiation therapy was even more effective, causing pancreatic tumors in the mice to shrink.
Findings from the NCI-supported study, led by David DeNardo, Ph.D., of the Washington University School of Medicine in St. Louis, were reported March 16 in Cancer Cell.
These findings “have a strong potential to be rapidly translated into novel treatment strategies for pancreatic cancer, a highly lethal malignancy,” said Serguei Kozlov, Ph.D., of the Frederick National Laboratory for Cancer Research, an expert on the interplay between the immune system and pancreatic cancer who was not involved in the study.
Further studies are needed to closely examine the safety of the dendritic cell–directed treatment, as well as its efficacy against cancer that has spread beyond the pancreas, Dr. Kozlov added.
Neoantigens Accelerate Pancreatic Cancer Growth
The immune system has a remarkable ability to kill abnormal or infected cells while leaving healthy cells alone. It tells healthy and diseased cells apart by scanning proteins called antigens on the surface of cells.
If the antigens on a cell’s surface appear normal, the immune system recognizes the cell as part of the body and leaves it alone. But if the antigens are unfamiliar or abnormal (what are called neoantigens), the immune system is more likely to attack.
Scientists think that immunotherapies work best for people whose tumors contain many neoantigens and cancer-killing immune cells. Pancreatic tumors have neoantigens—although not as many as lung or skin cancer—and some have cancer-killing immune cells in them, Dr. DeNardo explained.
So why don’t immunotherapies work for people with pancreatic cancer? One theory is that something in the environment around pancreatic cancer cells prevents the immune system from attacking.
To find out whether that might be the case, Dr. DeNardo’s team modified two well-established mouse models of pancreatic and lung cancer. Both models closely mimic how these cancers are thought to develop in humans, but they don’t have enough neoantigens for the immune system to spring into action.
To address this, the scientists engineered the cancer cells of both models to express an artificial neoantigen, a widely studied protein from chicken eggs called ovalbumin.
This “elegant genetic approach … enables more accurate examination of antigen-specific immune responses in pancreatic cancer and how such responses affect cancer growth,” Dr. Kozlov said.
Dr. DeNardo’s team expected the neoantigen to trigger an immune response that slows tumor growth, which is exactly what they saw in the lung cancer model. But, in the pancreatic cancer model, tumors that had the neoantigen grew and spread faster than tumors that did not have the neoantigen.
Further experiments revealed that pancreatic tumors with neoantigen expression had more immune cells that help cancer cells survive and grow. These “pro-cancer” immune cells were present starting from the early stages of pancreatic cancer development, the researchers found.
Pancreatic Tumors Have Fewer, Less Active Dendritic Cells
Next, the team looked at the various types of immune cells in pancreatic and lung tumors with the neoantigen and found a glaring difference.
Pancreatic tumors had far fewer dendritic cells than lung tumors—almost 80 times less. Dendritic cells were also sparse in samples of pancreatic tumors from people, they discovered.
An important role for dendritic cells is teaching cancer-killing T cells what neoantigens to look for in a process called T-cell licensing. In lab studies, the researchers found that dendritic cells from pancreatic tumors were less able to present antigens. As a result, far fewer T cells from mice with pancreatic cancer recognized the artificial neoantigen.
If cancer-fighting T cells are inside the tumor but don’t recognize the tumor’s neoantigens, “then that might be a problem” and might explain why the immune response against pancreatic cancer is ineffective, Dr. DeNardo said.
One possible explanation for why there are fewer dendritic cells in pancreatic tumors is that a healthy pancreas may not have many dendritic cells patrolling through it in the first place, Dr. DeNardo said. Unlike the lungs or skin, the pancreas isn’t a “barrier” organ that encounters a lot of invaders, he explained, so it might not need a lot of dendritic cells.
Another possibility is that dense scar tissue, which typically surrounds pancreatic cancer cells, might prevent dendritic cells from reaching or surviving in the tumor, he added.
Boosting Dendritic Cells in Pancreatic Tumors
Based on these findings, the team reasoned that luring dendritic cells into pancreatic tumors might jump-start an immune response against the cancer. They turned to two drugs, one that mobilizes dendritic cells (called a Flt3 ligand) and another that enhances the function and survival of dendritic cells (called a CD40 agonist).
Treating mice with pancreatic cancer with the drug combination caused dendritic cells to flood into tumors. Compared with mice that were not treated or were treated with only one of the drugs, mice that were treated with the combination had many more cancer-killing T cells—including T cells that recognize the neoantigen—in their tumors. The combination treatment also slowed tumor growth.
Encouraged by these results, the researchers considered ways to enhance the effects of the drug combination. They turned to radiation therapy because studies have shown that it can trigger an anticancer immune response by killing cancer cells and releasing neoantigens.
Although radiation alone or the drug treatment alone had a small effect on tumor growth, treatment with the drug combination followed by radiation shrank pancreatic tumors in mice. And mice treated with the triple therapy lived longer than mice treated with radiation alone.
The team is exploring the effects of the dendritic cell-directed treatment in combination with an immune checkpoint inhibitor (a type of immunotherapy) in follow-up studies, Dr. DeNardo said.
Targeting the Innate Immune System
Dendritic cells are part of the “innate” immune system, the body’s initial response to infection or disease. After that, the “adaptive” immune system—which includes T cells and antibodies—kicks in.
Most immunotherapies in current use target components of the adaptive immune system. This study, on the other hand, is in line with a recent shift toward developing cancer treatments that target cellular components of the innate immune system that have a role in cancer, Dr. Kozlov said.
“These findings identify dendritic cells as yet another component of the innate immune system that, if targeted therapeutically, may improve cancer outcomes,” he noted.
And it’s possible that “targeting parts of the innate and adaptive immune systems at the same time … could lead to additional advances in cancer treatment,” Dr. Kozlov added.
The study also sparks hope that treatments targeting dendritic cells might be effective against other types of cancer that don’t typically respond to existing immunotherapies, he said.