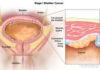
Allergan is recalling all of its BIOCELL textured breast implant products worldwide, after a request from the FDA to remove them from the market in the U.S. Their textured implants have been linked to an increased risk of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL).BIA-ALCL is a type of non-Hodgkin’s lymphoma (cancer of the immune system) — it is not breast cancer. BIA-ALCL may occur years after implant surgery, and is typically found in the scar tissue and fluid around the implant.Common symptoms include persistent swelling or pain in the area of the breast implant, a lump under the skin, breast asymmetry, and other breast changes, often years after the implant has been placed and healed.The risk of developing BIA-ALCL is low, and can be treated successfully in most patients by removing the implant and surrounding scar tissue. However, the cancer is serious, and some patients may also need chemotherapy and radiation. The disease can even turn deadly if it is not caught early enough.
 Photo: Wikimedia Commons/FDAAwareness about the cancer risk associated with some implants has been increasing as methods of reporting have become more robust. In February of 2019, there were 457 cases of BIA-ALCL worldwide along with 24 deaths. By July 2019, those numbers increased significantly. There have now been 573 unique cases of BIA-ALCL, 481 (or 84%) of which have been attributed to Allergan implants specifically.There have also been more deaths — that number now sits at 33 patients. Of the 13 patients who had a confirmed manufacturer of their implants, 12 of them had Allergan breast implants at the time of their cancer diagnosis.In the U.S., the risk of developing BIA-ALCL with Allergan BIOCELL textured implants is roughly six times the risk of developing it from other manufacturers’ textured implants.
Photo: Wikimedia Commons/FDAAwareness about the cancer risk associated with some implants has been increasing as methods of reporting have become more robust. In February of 2019, there were 457 cases of BIA-ALCL worldwide along with 24 deaths. By July 2019, those numbers increased significantly. There have now been 573 unique cases of BIA-ALCL, 481 (or 84%) of which have been attributed to Allergan implants specifically.There have also been more deaths — that number now sits at 33 patients. Of the 13 patients who had a confirmed manufacturer of their implants, 12 of them had Allergan breast implants at the time of their cancer diagnosis.In the U.S., the risk of developing BIA-ALCL with Allergan BIOCELL textured implants is roughly six times the risk of developing it from other manufacturers’ textured implants.
 Allergan is recalling the following BIOCELL textured breast implant products:Natrelle Saline-Filled breast implants – formerly McGhan RTV Saline-Filled Mammary Implant (P990074)Style 163 – BIOCELL Textured Shaped Full Height, Full Projection Saline Breast ImplantsStyle 168 – BIOCELL Textured Round Moderate Profile Saline Breast Implants, also referred to as 168MP (168 Moderate Profile)Style 363 – BIOCELL Textured Shaped Moderate Height, Full Projection Saline Breast Implants, Allergan catalog includes 363LF, or 363 Low Height Full ProjectionStyle 468 – BIOCELL Textured Shaped Full Height Moderate Projection Saline Breast Implants
Allergan is recalling the following BIOCELL textured breast implant products:Natrelle Saline-Filled breast implants – formerly McGhan RTV Saline-Filled Mammary Implant (P990074)Style 163 – BIOCELL Textured Shaped Full Height, Full Projection Saline Breast ImplantsStyle 168 – BIOCELL Textured Round Moderate Profile Saline Breast Implants, also referred to as 168MP (168 Moderate Profile)Style 363 – BIOCELL Textured Shaped Moderate Height, Full Projection Saline Breast Implants, Allergan catalog includes 363LF, or 363 Low Height Full ProjectionStyle 468 – BIOCELL Textured Shaped Full Height Moderate Projection Saline Breast Implants
Natrelle Silicone-Filled breast implants – formerly Inamed Silicone-Filled Breast Implants (P020056)Style 110 – BIOCELL Textured Round Moderate Projection Gel Filled Breast ImplantsStyle 115 – BIOCELL Textured Round Midrange Projection Gel Filled Breast ImplantsStyle 120 – BIOCELL Textured Round High Projection Gel Filled Breast ImplantsStyles TRL, TRLP, TRM, TRF, TRX – Natrelle Inspira BIOCELL Textured Responsive Silicone-Filled Breast ImplantsStyles TCL, TCLP, TCM, TCF, TCX – Natrelle Inspira BIOCELL Textured Cohesive Silicone-Filled Breast ImplantsStyles TSL, TSLP, TSM, TSF, TSX – Natrelle BIOCELL Textured Soft Touch Silicone-Filled Breast ImplantsNatrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants (P040046)Styles 410FM, 410FF, 410MM, 410 MF, 410 FL, 410 ML, 410 LL, 410 LM, 410 LF, 410 FX, 410 MX, 410 LXNatrelle 133 Plus Tissue Expander (K143354)Natrelle 133 Tissue Expander with Suture Tabs (K102806)
 Photo: Adobe Stock/YakobchukOlenaThe FDA first took notice of BIA-ALCL in in 2011. As the years went on, more and more cases cropped up. In late 2018, health officials in France asked Allergan to recall their textured breast implants due to its cancer risk; their implants make up about 85% of cosmetic and reconstructive implants in France. Allergan stopped selling their implants and expanders in the rest of Europe, pulling them from the market in 33 countries.From there, beginning in April of 2019, France banned all textured implants. It targeted six manufacturers in particular: Allergan, Arion, Sebbin, Nagor, Eurosilicone, and Polytech.Textured implants only make up about 10% of the U.S. implant market. About 10 million women worldwide have breast implants of any kind.
Photo: Adobe Stock/YakobchukOlenaThe FDA first took notice of BIA-ALCL in in 2011. As the years went on, more and more cases cropped up. In late 2018, health officials in France asked Allergan to recall their textured breast implants due to its cancer risk; their implants make up about 85% of cosmetic and reconstructive implants in France. Allergan stopped selling their implants and expanders in the rest of Europe, pulling them from the market in 33 countries.From there, beginning in April of 2019, France banned all textured implants. It targeted six manufacturers in particular: Allergan, Arion, Sebbin, Nagor, Eurosilicone, and Polytech.Textured implants only make up about 10% of the U.S. implant market. About 10 million women worldwide have breast implants of any kind.
![]() Photo: Adobe Stock/branislavpIn May of 2019, the FDA announced it would allow the implants to remain on the market in the U.S., though they were considering giving the products bold warnings.But barely two months later, in July of 2019, new information made them reconsider.“Based on new data, our team concluded that action is necessary at this time to protect the public health,” said FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D. “We will continue to monitor the incidence of BIA-ALCL across other textured and smooth breast implants and tissue expanders as well as other devices intended for use in the breast. If action is needed in the future, we will not hesitate to do what is necessary to protect patients.”If you have textured implants but aren’t experiencing any symptoms, there’s no need to get the implants removed at this time. This recall is intended to ensure that all unused products are removed from both suppliers and doctors’ offices.Source
Photo: Adobe Stock/branislavpIn May of 2019, the FDA announced it would allow the implants to remain on the market in the U.S., though they were considering giving the products bold warnings.But barely two months later, in July of 2019, new information made them reconsider.“Based on new data, our team concluded that action is necessary at this time to protect the public health,” said FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D. “We will continue to monitor the incidence of BIA-ALCL across other textured and smooth breast implants and tissue expanders as well as other devices intended for use in the breast. If action is needed in the future, we will not hesitate to do what is necessary to protect patients.”If you have textured implants but aren’t experiencing any symptoms, there’s no need to get the implants removed at this time. This recall is intended to ensure that all unused products are removed from both suppliers and doctors’ offices.Source