October 23, 2018, by NCI Staff
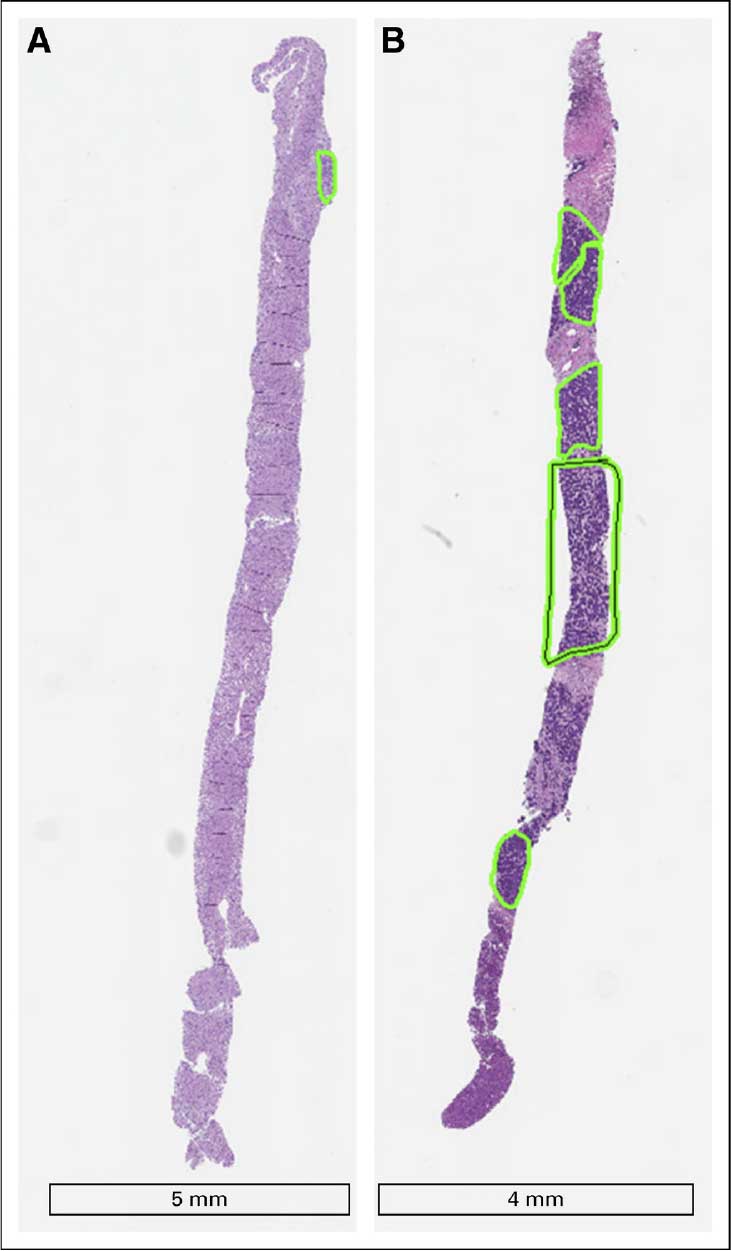
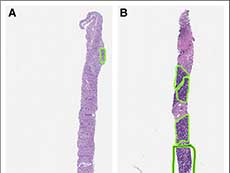
Tumor biopsies collected for research often don’t have enough of the tumor tissue. These two biopsy samples from the same patient have very different amounts of tumor tissue (green boxes).
Reprinted with permission J Oncol Pract. 2018 Oct 4:JOP1800092.
Researchers have developed recommendations for improving the quality of biopsy specimens collected from patients participating in cancer clinical trials. Tumor biopsy samples can provide valuable information to researchers, such as insights into how cancer therapies behave in the human body.
The recommendations provide practical suggestions for the leaders of clinical trials, such as ensuring that the interventional radiologists who collect the study biopsy specimens are given clear information about the study’s goals and tissue requirements. Interventional radiologists use ultrasound, CT scans, and MRI to identify locations in tumors from which they extract tissue.
Other recommendations include increasing the amount of biological material collected for research and ensuring that patients who are enrolled in a clinical trial that has a requirement for a research biopsy have tissue that is amenable to biopsy.
The recommendations, which appeared in the Journal of Oncology Practice on October 4, were based on an analysis of research biopsies conducted by a multidisciplinary team of experts.
“Our analysis suggests that clear communication by all parties involved in the collection, handling, processing, and storing of biopsies is critical for success,” said Kate Ferry-Galow, Ph.D., of the Clinical Pharmacodynamic Biomarkers Program at NCI’s Frederick National Laboratory for Cancer Research.
“We now hold regular meetings throughout a clinical trial, and the biopsy team—including the oncologists, interventional radiologists, pathologists, and assay scientists—communicate about the quality of the biopsies,” Dr. Ferry-Galow continued. “That communication has helped tremendously.”
When the researchers implemented the recommendations during clinical trials conducted by NCI’s Developmental Therapeutics Clinic, the new strategy led to a better success rate in terms of obtaining suitable biopsy specimens for analysis, compared with previous studies.
Using Tumor Samples to Conduct Multiple Tests
Conducting biopsies for research purposes can be challenging, Dr. Ferry-Galow noted. One challenge is that such biopsies ideally involve the collection of more tissue from a patient than biopsies conducted during routine patient care.
As the team’s analysis revealed, the need for additional tissue has not always been communicated clearly to interventional radiologists on clinical trials. The need for more tissue reflects the fact that research biopsy specimens may be used for a variety of genetic and molecular tests, such as next-generation DNA sequencing.
In some early-phase clinical trials of investigational therapies, for example, researchers may collect tissue from the same tumor before and after a patient receives a drug. Analyzing pairs of biopsy specimens can reveal whether drugs designed to block certain signaling pathways in tumors actually do so in the human body.
“Today, we can develop evidence that a drug is working at the molecular level—and in greater detail than ever before,” said coauthor Alda Tam, M.D., the clinical medical director in the Department of Interventional Radiology at the University of Texas MD Anderson Cancer Center.
Despite the importance of research biopsies, these procedures often fail to produce the specimens needed to answer research questions about cancer clinical trials, Dr. Tam noted.
A retrospective analysis of four early-phase clinical trials conducted at NCI’s Developmental Therapeutics Clinic, for example, found that only 83 of 112 (74%) needle biopsies collected to study the activity of drugs in the body produced specimens that met quality-control standards. The reasons included an insufficient amount of tumor collected and/or specimens of poor quality.
Bringing Together Experts on Research Biopsies
To address the need to improve research biopsies, Alice Chen, M.D., of NCI’s Division of Cancer Treatment and Diagnosis (DCTD), and her colleagues convened several meetings with oncologists, pathologists, laboratory scientists, and interventional radiologists from around the country. The participants reviewed and discussed all aspects of the processes involved in collecting and analyzing research biopsies.
The discussions “represent the first time we’ve had a national conversation about research biopsies that included interventional radiologists and pathologists,” said Dr. Tam. “And the conversation showed that we all could work better as a team.”
Among other findings, the researchers learned that some radiologists weren’t aware of the different requirements for research biopsies compared with the requirements for other types of biopsies, such as diagnostic biopsies, Dr. Ferry-Galow said.
Another finding was the need for greater recognition and financial support for the investment of time and scientific contributions made by interventional radiologists and pathologists.
“What interventional radiologists are asked to do in the research setting is different from what they do in the clinical setting, and they aren’t always being recognized for their time,” said Dr. Ferry-Galow.
The study authors suggested that the important contributions of interventional radiologists and pathologists could be recognized by making them coauthors of studies reporting the results of clinical trials they helped to conduct.
Sharing the Findings with the Research Community
As a next step, the study authors are working to communicate the need and the resources available for improving the quality of research biopsy specimens. For example, DCTD posts standard operating procedures for tissue collection for validated biomarker assays online.
In addition, the publicly available Biospecimen Research Database—which includes standard operating procedures in the field of human biospecimen science—is being revised as a resource to promote the dissemination of references, data, and best practices related to radiology and pathology, the study authors wrote.
They plan to conduct additional outreach at national scientific meetings and through professional societies.
“The findings of our study are incredibly positive,” said Dr. Tam. “They show that just sitting down and talking through what each person brings to a clinical trial will improve the execution of the biopsy process and quality of the results.”