, by Carmen Phillips
Treatment options for people with the aggressive blood cancer acute myeloid leukemia (AML) have expanded yet again with a new approval from the Food and Drug Administration (FDA).
On July 20, the agency approved quizartinib (Vanflyta) combined with chemotherapy as part of the initial treatment of people with AML that has a specific change in a gene called FLT3.
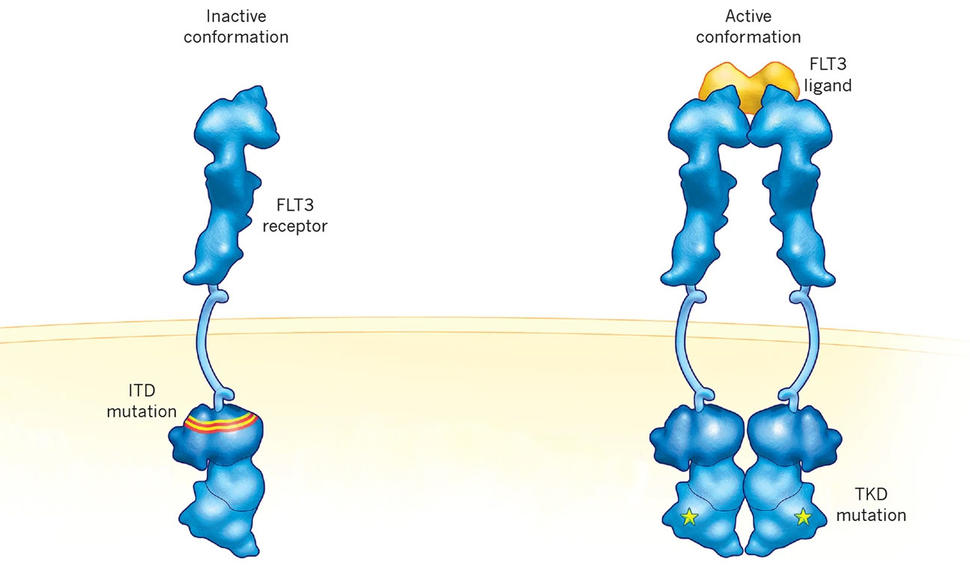
Genetic changes in FLT3 are common in people diagnosed with AML. The FLT3 mutation covered by the approval, known as an internal tandem duplication (ITD), is present in about one-quarter of people diagnosed with this cancer.
FDA’s approval was based on results from a large clinical trial called QuANTUM-First. In the trial, which included more than 500 people with this specific form of AML, participants who received quizartinib plus standard chemotherapy lived more than twice as long as those who received standard treatment alone: a median of 32 months versus 15 months. Results from the trial were published in May in The Lancet.
The agency also approved a blood test to identify patients with the FLT3 ITD mutation. Such tests are called companion diagnostics.
In rare cases, quizartinib can cause potentially fatal changes in heart function. So, under the approval, the drug must be prescribed as part of a special FDA safety program called Risk Evaluation and Mitigation Strategies (REMS).
FDA’s decision makes quizartinib the second FLT3-targeted drug approved as an initial, or first-line, treatment for people with AML. The first, midostaurin (Rydapt), was approved in 2017. A third drug, gilteritinib (Xospata), has also been approved to treat AML, but only for people whose cancer has come back after earlier treatment, known as second-line treatment.
AML experts generally agreed that the approval of quizartinib offers an important new option for people whose cancer has the ITD mutation in FLT3. Several noted that the trial results are particularly noteworthy because it included many participants over the age of 60. More than half of AML diagnoses are in people in this age group.
By comparison, midostaurin’s approval was based on a trial that didn’t include any patients older than age 59.
For people with the FLT3 ITD mutation, including older patients, the new approval “is a big step forward,” said Harry Erba, M.D., of the Duke University Cancer Institute and one of the trial’s lead investigators.
As long as the person is well enough to receive the intensive chemotherapy included in the treatment, Dr. Erba said, the addition of quizartinib “should become the standard of care for patients with FLT3 ITD-positive AML.”
Attacking AML with FLT3 mutations
AML is one of the most aggressive forms of leukemia. When the AML has certain genetic changes in FLT3, it becomes even more troublesome, increasing the likelihood that the cancer will come back after initial treatment and making it less likely to respond to other treatments.
Midostaurin’s approval in 2017 (in combination with chemotherapy) and the earlier use of stem cell transplants have helped people with AML with FLT3 mutations live much longer than they once would have, wrote Amanda Przespolewski, M.D., and Elizabeth Griffiths, M.D., in a Lancet editorial that accompanied the trial results.
There was no age restriction on FDA’s approval of midostaurin. But given the limited data on its use in people 60 and older, Drs. Przespolewski and Griffiths wrote, “most patients might be
excluded from [receiving] this effective additional therapy.”
Although both midostaurin and quizartinib target cancer cells with FLT3 mutations, quizartinib was designed specifically to target AML cells with the ITD mutation in FLT3. Midostaurin and gilteritinib, on the other hand, also target another mutation in FLT3 called TKD.
Doubling of overall survival with the addition of quizartinib
The QuANTUM-First trial—funded by Daiichi-Sankyo, which makes quizartinib—enrolled 539 adults, ranging in age from 18 to 75. About 40% of participants were 60 or older. Many participants also had mutations in another gene, NPM1. The negative impact of the FLT3 ITD mutation is most pronounced in people with AML that also has NPM1 mutations, Dr. Erba said.
Drs. Przespolewski and Griffiths applauded the trial leaders for enrolling “a more realistic patient population” than other trials involving people with AML.
Participants in the trial who were randomly assigned to the quizartinib group received the drug along with chemotherapy as part of the standard two-step treatment process. Part one is called induction, which is intended to send the AML into remission, and part two is called consolidation, to help sustain that remission. The other participants received chemotherapy and a placebo on the same schedule.
Consolidation therapy in people with AML can be an allogeneic stem cell transplant. In people with FLT3 ITD mutations, such transplants are a standard consolidation treatment for those who are healthy enough to receive one, Dr. Erba said. A similar number of participants in both the quizartinib and placebo groups received transplants as consolidation therapy.
Patients in the quizartinib group continued taking the drug for up to 3 years as maintenance therapy, a further step to keep the disease from returning.
Although there was a doubling of overall survival in the quizartinib group, the improvement in how long participants in this group lived was most substantial in those under age 60.
But treatment with quizartinib had other clinically important benefits, Dr. Erba said.
For example, although patients in both groups had similar rates of complete remissions after induction therapy and consolidation therapy, the remissions were substantially longer among people treated with quizartinib: a median of 38.6 months versus 12.4 months.
Treatment with quizartinib also helped people live longer regardless of whether they underwent a stem cell transplant.
Finally, for a group of patients in the trial, the research team used a sophisticated technique, called next-generation sequencing, to assess whether participants who had a complete remission still had any detectable leukemia cells with the FLT3 ITD mutation in their bone marrow.
When no such cells can be detected, it’s called being minimal residual disease (MRD)-negative.
Regardless of which treatment they received, people who were MRD-negative lived longer overall than those who were not MRD-negative. It’s unclear how much longer, however, because not enough participants who were MRD-negative had died yet to determine a median overall survival, while the median overall survival for the MRD-positive group was about 27 months.
This finding, Dr. Erba said, points to the potential of using MRD status after induction therapy to help guide further treatment decisions.
Farhad Ravandi, M.D., who specializes in treating AML at the University of Texas MD Anderson Cancer Center and was not involved in the trial, agreed. “MRD assessment by sensitive next-generation [sequencing] is increasingly important in this and other types of leukemia,” Dr. Ravandi said.
Side effects and REMS
People in the quizartinib group were more likely than those in the chemotherapy-alone group to have serious treatment-related side effects, most commonly substantial drops in white blood cell counts. And slightly more people treated with quizartinib died during the first 2 months of treatment. Most deaths were caused by infections.
Midostaurin tends to cause substantial gastrointestinal side effects, such as nausea and
diarrhea. But, in the QuANTUM-First trial, rates of these side effects were very low in people treated with quizartinib, Dr. Erba explained.
A heart-related side effect called prolonged QT interval was more common in people treated with quizartinib, which led FDA to require oncologists to go through the REMS process to prescribe the drug. This side effect involves changes to the heart’s electrical system that can affect its normal rhythm. In a small percentage of people, this problem can be fatal.
In the trial, about 2% of people in the quizartinib group had more severe forms of the condition, compared with 1% in the chemotherapy-alone group.
In earlier studies of quizartinib, prolonged QT interval was seen in some patients given higher doses of the drug, Dr. Erba said. But the lower dose used in the QuANTUM-First trial and approved by FDA causes this problem less frequently and when it does occur, it is usually less severe, he said.
Going through the REMS process “could be an impediment to [oncologists] prescribing quizartinib,” he acknowledged. “But I believe the benefit of this drug is significant and should outweigh the extra step needed to meet the REMS requirement.”
Which FLT3 targeted drug to use?
A challenge for oncologists now is deciding which FLT3-targeted drug to use in which patients.
Quizartinib’s approval is only for the initial treatment of people whose tumors have the FLT3 ITD mutation. And although midostaurin can be used in people with either the ITD or TKD mutation, Dr. Ravandi said that he expects many oncologists who regularly treat AML will likely use quizartinib as the preferred first treatment in patients with the ITD mutation.
In addition to the fact that it specifically targets this mutation, Dr. Erba highlighted another key reason for preferring it to midostaurin: When people with the ITD mutation experience a relapse, it’s often due to the development of the TKD mutation.
“So it makes sense to use quizartinib first” and reserve gilteritinib, which is specifically approved as a second-line treatment, for situations where the relapse is caused by a TKD mutation, he said.
There’s also the question of whether patients need to stay on quizartinib maintenance treatment for 3 years. In the clinical trial, the median time participants stayed on the drug was just 16 months, with most discontinuing because of side effects.
Dr. Ravandi acknowledged that the cost of maintenance treatment and dealing with side effects “can be a concern,” but said he would advocate for patients staying on quizartinib for the full treatment period, if they can.
But that is a decision, he continued, where, as more studies are done, “MRD assessment may become a deciding factor.”