, by NCI Staff
The Food and Drug Administration (FDA) has approved a combination of two immunotherapy drugs for the treatment of some people with advanced melanoma. The combination consists of relatlimab and nivolumab (Opdivo) and will be marketed under the name Opdualag.
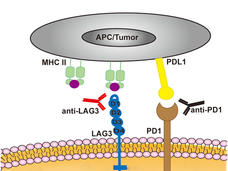
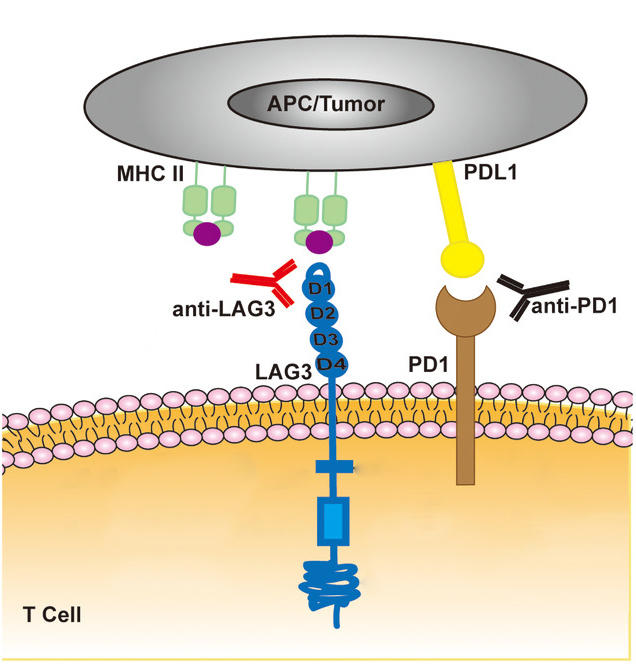
Both drugs are immune checkpoint inhibitors, which target proteins called checkpoints that help stop the immune system from mounting a strong response against cancer cells. Relatlimab blocks a protein on immune cells called LAG-3, while nivolumab blocks a different protein on immune cells called PD-1. By blocking these proteins, these drugs can unleash an immune response against cancer cells.
Relatlimab is the first FDA-approved drug to block the activity of LAG-3.
Unlike other FDA-approved combinations of immune checkpoint inhibitors, with Opdualag patients receive an intravenous infusion containing both drugs.
FDA approved the combination of nivolumab and relatlimab for people aged 12 or older with previously untreated melanoma that cannot be removed surgically or has spread (metastasized) within the body.
The approval was based on results from a large clinical trial called RELATIVITY-047. This study compared the combination of nivolumab and relatlimab with nivolumab alone, which is a standard treatment for patients diagnosed with inoperable or metastatic melanoma.
Bristol Myers Squibb, which manufactures nivolumab and relatlimab, sponsored the trial. The researchers measured how long patients lived without their disease worsening or dying from any cause, whichever came first (progression-free survival).
With a median follow-up of 13.2 months, patients who received nivolumab and relatlimab had longer progression-free survival than patients who received nivolumab alone (10.1 months versus 4.6 months).
Among the patients who received nivolumab and relatlimab, the most common side effects were fatigue, rash, aching or painful joints, and diarrhea. Of this group, 14.6% of the patients stopped treatment because of side effects, compared with 6.7% of the nivolumab-alone group.
“The results of this study are quite impressive,” said Elad Sharon, M.D., who helps lead immunotherapy trials in NCI’s Cancer Therapy Evaluation Program but was not involved in the research. Dr. Sharon cautioned, however, that longer follow-up is needed to show whether the new combination improves how long patients live (overall survival), compared with other treatments.
Expanding the number of immune checkpoint inhibitors
Additional drugs targeting LAG-3 are being evaluated for the treatment of multiple myeloma, esophageal or gastric cancer, and chordoma, among other types of cancers. And Opdualag is also being studied in clinical trials of other cancers, including lung, colorectal, and liver cancer.
The NCI-MATCH trial recently added a new treatment group to evaluate Opdualag in patients whose cancers have progressed after treatment with immune checkpoint inhibitors that target PD-1 or its binding partner on cancer cells, PD-L1.
Combinations of immune checkpoint inhibitors may be more effective than an individual immune checkpoint inhibitor for treating certain cancers, studies have shown. For example, nivolumab and ipilimumab (Yervoy), which blocks a checkpoint protein on immune cells called CTLA-4, is more effective than nivolumab alone for treating melanoma that has spread to the brain.
The combination of nivolumab and ipilimumab is a standard treatment for previously untreated inoperable or metastatic melanoma. In terms of progression-free survival, the combinations of nivolumab plus ipilimumab and of relatlimab plus nivolumab have yielded similar results in clinical trials.
However, the relatlimab–nivolumab regimen appears to be associated with fewer side effects than the nivolumab–ipilimumab regimen. Less than 20% of patients who received relatlimab–nivolumab reported serious side effects, compared with nearly 60% patients who received nivolumab–ipilimumab, noted the authors of an editorial accompanying the RELATIVITY-047 results in the New England Journal of Medicine.
“Given the different safety profiles of these regimens,” said Dr. Sharon, “it will be interesting to see over time if the nivolumab and relatlimab combination improves overall survival, compared with nivolumab and ipilimumab.”
Longer follow-up data from the RELATIVITY-047 trial, he added, are needed to help patients and physicians select from various treatment options for advanced melanoma.
Results from the trial and their potential implications for patients were discussed in a June 2021 Cancer Currents story.