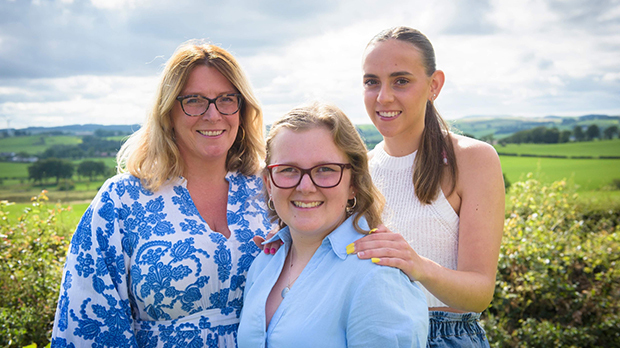 Children’s cancer mortality has almost halved in the UK in the past 3 decades*.
Children’s cancer mortality has almost halved in the UK in the past 3 decades*.
But we’ve made more progress in the treatment of some cancers than others.
Acute Lymphoblastic Leukaemia (ALL), the most common type of leukaemia diagnosed in children, has particularly high survival. In fact, thanks to major advances in treatment, more than 9 in 10 children in the UK with ALL now survive for at least 5 years, compared with around 7 in 10 in the 1980s.
By the early 2000s survival for children with this cancer type had already seen huge improvements. However, outcomes for children and young people who relapsed has remained static.
At the time, Vaskar Saha, based at the University of Manchester, was researching ways to improve treatment options for those that had relapsed.
Approximately 176 miles North, in East Kilbride, Katie Currie was diagnosed with ALL when she was just 3 years old. Following successful treatment, in 2008, her cancer came back. She was put onto a trial, led by Saha, for a chemotherapy drug known as mitoxantrone.
In an unusual turn of events, the results of mitoxantrone turned out to be so effective that eventually all the children who were recruited for the trial were put on the drug.
The ALLR3 trial
“At that time, there was really no straightforward standard pathway for treating children with relapsed acute lymphoblastic leukaemia. But there were many new things happening in the research” explains Saha, now Head of Paediatric Haematology and Oncology at the Tata Medical Center, Kolkata.
“One important thing was the discovery that you could monitor the response to treatment using molecular tools, what we call minimal residual disease (MRD), where you can detect one in 10,000 cells.”
And at the same time as MRD was being developed, paediatric oncologists worldwide were beginning to work together. “Scientists across Europe, Australia and New Zealand put together a study, which was testing whether MRD could actually tell us whether one drug was going to do better than another.”
The international team set up a trial, known as ALLR3, comparing idarubicin, a chemotherapy treatment which was the standard of care for relapsed ALL, to mitoxantrone, which had shown promise in the lab.
216 children and young people took part in this trial and were split into 2 groups who were given either idarubicin or mitoxantrone, alongside other standard chemotherapy drugs.
“We took it day by day, especially after she relapsed,” recalls Katie’s Dad Neil. “When the cancer came back, you fear everything, but we spoke to the team about the trial and put complete faith in them.”
3 years on from the treatment, the researchers looked at the early results to see how the treatment was working.
What happened next was something that had never been seen in a trial for childhood ALL.
A surprising set of results
The study evaluated the number of children whose leukaemia hadn’t got worse over the 3 years since treatment (known as progression free survival). Results showed that over 6 out of 10 children (64.6%) who had mitoxantrone, compared to less than 4 out of 10 children (35.9%) who had idarubicin, had cancers that hadn’t grown at all in the timeframe.
The study also found that the number of children alive 3 years after treatment, regardless of whether their cancer had grown or not, was far higher in the group on mitoxantrone compared to idarubicin (known as overall survival).
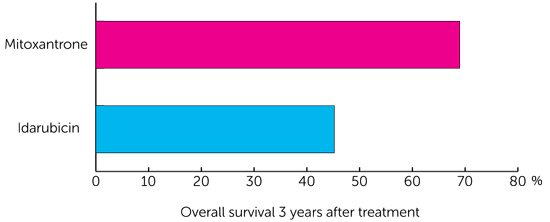
“I kind of knew that the arms were moving apart in terms of survival outcomes,” says Saha. “But then I got a call from Professor Mike Stevens, from Bristol University who was the chairALLR3 and he said, ‘You need to close the randomisation as there is a significant difference in outcome between the two drugs.’”
The randomisation arms of the trial were halted early, and from then on, all the children who were originally on idarubicin were transferred to mitoxantrone.
A difference in outcomes like that had never been reported before. It took all of us by surprise.
– Vaskar Saha, lead researcher on the mitoxantrone trial
Interestingly, the two drugs showed no difference in minimal residual disease, which is what the experts were initially expecting to see. “It showed very clearly that you can’t use MRD as a surrogate marker for outcome, which impacted the design of similar trials subsequent to these results.”
The real life impact
Katie thinks back to the period of her diagnosis. “I was so young when I was diagnosed that I don’t remember much from then, but I do remember being in hospital and I remember the nurses. It is surreal to think about what I went through then and how far I have come.”
Thanks to the trial headed by Saha, Katie is embarking on her career in medicine, starting child health nursing at university.
I knew I wanted to do something in medicine after what had happened to me. By doing this, I really want to say thank you for the help I had, and I am passionate about nursing.
– Katie
When she got her Higher exam results, she was delighted to be offered a place on a course at Edinburgh Napier University. “I finished school in 2020 but it was a slightly difficult time then as I was on the list for shielding due to COVID-19, so I deferred a year before starting uni. I had a year out and went to college to get another qualification – an HNC in Healthcare.”
Katie started her course in September last year, and despite having to continue to shield at the beginning of her course, is really enjoying her time there. “I have been on two placements already and I think that my own experience has really helped me with my understanding with patients.”
“Thinking back to those days of Katie’s treatment and relapse when she was so young, it is just amazing to be here now and for her to be away in Edinburgh following her dreams,” says Neil.
“We believed that, with the clinical trial Katie had the best chance of recovery. Without these trials, amazing new treatments may never be found. Mitoxantrone probably saved Katie’s life,” adds Mum Siobhan.
Saha recalls the extraordinary impact this study also had on the research landscape. “This publication really took the whole field forward in terms of what drugs you could use to treat childhood ALL. Even now, 20 years on, the backbone of the chemotherapy that we used in the trial is being modified and used by other people.”
Lilly
September is Childhood Cancer Awareness Month. Each year in the UK, around 4,200 children and young people are diagnosed with cancer**. We’re determined to overcome the challenges holding back progress, to help more 0-24-year-olds with cancer survive with a good quality of life. We’re working to develop a strong, long-lasting community of children’s and young people’s cancer researchers and providing the tools and infrastructure this community needs to accelerate progress.
Find out more about our research into cancers affecting children and young people or follow us on facebook for news and updates on our work in this area.
* Based on the percentage change of world age-standardised mortality rate from cancer (C00-C97, D32-D33, D35.2-D35.4, D42-D43, D44.3-D44.5) in children aged 0-14 years, between 1987-89 and 2017-19’
** Based on the average annual number of new cases of cancer (C00-C97, D32-D33, D35.2-D35.4, D42-D43, D44.3-D44.5) in the UK in children and young people aged 0-24 years, between 2016-2018.