, by NCI Staff
In what many global health leaders are calling a milestone study, researchers in Sweden have confirmed that widespread use of the human papillomavirus (HPV) vaccine dramatically reduces the number of women who will develop cervical cancer.
In the study of nearly 1.7 million women, the vaccine’s efficacy was particularly pronounced among girls vaccinated before age 17, among whom there was a nearly 90% reduction in cervical cancer incidence during the 11-year study period (2006 through 2017) compared with the incidence in women who had not been vaccinated.
“This is a vaccine against cancer, which can save lives,” said the study’s leader, Jiayao Lei, Ph.D., of the Karolinska Institute in Stockholm.
On Twitter, Noel Brewer, Ph.D., who studies cancer prevention and HPV vaccines at the University of North Carolina, called the study results “incredibly powerful.” The findings were published September 30 in the New England Journal of Medicine.
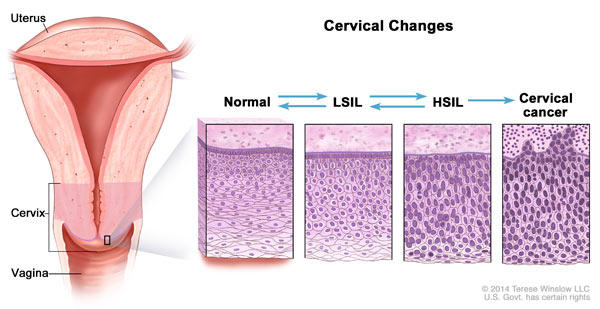
Studies and clinical trials to date have consistently shown that HPV vaccines are extremely effective at reducing infections with the types of the virus that can lead to cancer, as well as cervical precancers. But because of the long time between infection and cancer, it had yet to be shown that HPV vaccination prevents cervical cancers.
“Because HPV vaccination prevents persistent HPV infection and cervical precancer, the precursors to cervical cancer, we were confident we would eventually observe that HPV vaccination prevents cervical cancer. We also knew it would take time to observe that,” said Aimée R. Kreimer, Ph.D., of NCI’s Division of Cancer Epidemiology and Genetics, who studies HPV vaccines and cancer prevention.
“Cervical cancer can be a devastating diagnosis,” said Abbey Berenson, M.D., Ph.D., who specializes in women’s health at the University of Texas Medical Branch. The study results, Dr. Berenson continued, “send an incredibly important message” about the impact widespread use of the HPV vaccine can have.
The Missing Piece of Evidence
Large clinical trials of HPV vaccines—which enrolled thousands of participants and followed them over time—assessed their ability to prevent cervical infections with cancer-causing types of HPV and the development of precancerous lesions in the cervix that can result from those infections.
The clinical trials did not measure whether the vaccine prevents cervical cancer because precancerous lesions in the cervix found during a clinical trial would be treated, preventing their progression to cancer, Dr. Kreimer explained.
The Swedish study, however, looked back in time at a huge population of women. And for their study, the Swedish researchers had two factors in their favor: the individual-level data in the country’s nationwide public health registry and the fact that, beginning in 2007, the country has conducted a series of nationwide HPV vaccination programs.
The Swedish study isn’t the first large population-based study of HPV vaccines. In Australia, for example, researchers have shown that the country’s universal HPV vaccination program, launched in 2007, led to massive declines in infections with the HPV types covered by the vaccine, while also protecting against HPV infections in unvaccinated people, a phenomenon known as herd immunity.
It’s logical to conclude that if the vaccine slashes infections with cancer-causing HPV types and the development of advanced precancerous cervical lesions in women, then far fewer diagnoses of invasive cervical cancers should follow in the ensuing years, Dr. Berenson said.
Nevertheless, no studies had gone on long enough to deliver that logic to its anticipated result.
For HPV Vaccination: The Younger, the Better
The Swedish study is the largest to compare cervical cancer diagnoses among women who did and did not receive an HPV vaccine. In Sweden, the only HPV vaccine available during the time period studied was one that protects against four HPV types: HPV 6, HPV 11, HPV 16, and HPV 18. Infections with types 16 and 18 are responsible for approximately 70% of cervical cancers, and types 6 and 11 cause 90% of genital warts.
All of the females followed in the study were between the ages of 10 and 30. Approximately 528,000 of them had received at least one dose of the vaccine between 2006 and 2017, and the remaining 1.14 million had not been vaccinated. More than 80% of those vaccinated received the vaccine before they were 17 years old.
Overall, 19 of the vaccinated women were diagnosed with cervical cancer during the study period, compared with 538 of the unvaccinated women. After adjusting for different factors that can influence cervical cancer risk, those numbers translated into a 63% reduced risk of being diagnosed with cervical cancer among females who had been vaccinated compared with those who hadn’t.
The nearly 90% reduction in cervical cancer among women who were vaccinated at a younger age makes sense, said Dr. Kreimer.
Many women who received the vaccine after age 17 would be more likely to have HPV infections at the time of vaccination, and the vaccine only works to prevent infections, not stop existing infections. “Thus, the older girls were more likely to have had infections at the time of vaccination that were, therefore, not preventable and could progress to cancer.”
According to the study’s senior researcher, Pär Sparén, Ph.D., also of Karolinska, the findings affirm the need for broader use of the HPV vaccine among women in low- and middle-income countries, in which cervical cancer is often one of the leading causes of death.
“The evidence … highlights the importance of continuing to introduce HPV vaccination programs and maintaining a high [vaccine] coverage, preferably for girls at young age, to maximize the benefits,” Dr. Sparén said.
A Boost for Vaccinations?
The Swedish study does have some limitations. For example, it couldn’t account for factors such as the extent to which women followed in the study were screened for cervical cancer, the study team reported. The researchers also couldn’t capture the number of vaccine doses each person in the vaccination group received.
“But that’s not a big limitation for this type of study. It’s not a dose question,” Dr. Kreimer said. For this study, she continued, “They were saying, ‘We established a vaccine program in a population, and this is how [well] it worked.’”
Although rates of HPV vaccination have increased among adolescents and teens in the United States, they still are lower than public health officials would like. Dr. Berenson said she’s hopeful that the findings from the Swedish study can provide a boost.
“[The findings] provide a very good discussion point around age of vaccination,” she said. And that’s needed, she added, because parents sometimes are reluctant to have their daughters receive the HPV vaccine at the recommended age, which is 11-12 years old.
“They often tell us they want to wait until she’s older—until she’s 18—saying, ‘She can make the decision for herself,’” Dr. Berenson said. “This study gives [pediatricians] good evidence to say, ‘We understand why you may feel that way, but you are missing the opportunity of much higher efficacy if she gets vaccinated at a younger age.’”
It’s taken some time, but the findings from the Swedish study complete the story of the HPV vaccine, Dr. Kreimer said. “This provides the final key piece of evidence in the pathway from [HPV] infection to cancer,” she said, “and HPV vaccination protects against all of it.”