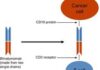
On 27 May 2022, the US Food and Drug Administration (FDA) granted accelerated approval to tisagenlecleucel (Kymriah, Novartis Pharmaceuticals Corporation) for adult patients with relapsed or refractory follicular lymphoma after two or more lines of systemic therapy.
The approval was based on the ELARA study (NCT03568461), a multicentre, single-arm, open-label study evaluating tisagenlecleucel, a CD19-directed chimeric antigen receptor (CAR) T cell therapy, in adult patients who were refractory or relapsed within 6 months after completion of two or more lines of systemic therapy (including an anti-CD20 antibody and an alkylating agent) or relapsed after autologous haematopoietic stem cell transplant. Following lymphodepleting chemotherapy, tisagenlecleucel was administered as a single intravenous infusion with a target dose of 0.6 to 6.0 x 108 CAR-positive viable T cells.
The main efficacy measures were overall response rate (ORR) and duration of response (DoR) as determined by an independent review committee. Among 90 patients in the primary efficacy analysis, the ORR was 86% (95% confidence interval [CI] 77, 92) with a complete response (CR) rate of 68% (95% CI 57, 77). The median DoR was not reached, with 75% (95% CI 63, 84) of responders still in response at 9 months. For all patients who underwent leukapheresis (n = 98), the ORR was 86% (95% CI 77, 92) with a CR rate of 67% (95% CI 57, 76).
The most common adverse reactions (>20%) occurring in patients were cytokine release syndrome, infection, fatigue, musculoskeletal pain, headache, and diarrhoea.
The recommended tisagenlecleucel dose is 0.6 to 6.0 x 108 CAR-positive viable T cells.
Full prescribing information for Kymriah is available here.
This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
This review used the Assessment Aid, a voluntary submission from the applicant to facilitate the FDA’s assessment. The FDA approved this application 1 month ahead of the FDA goal date.
This application was granted priority review and regenerative medicine advanced therapy designation. The application also was granted orphan drug designation.
Healthcare professionals should report all serious adverse events suspected to be associated with the use of any medicine and device to FDA’s MedWatch Reporting System.
For assistance with single-patient INDs for investigational oncology products, healthcare professionals may contact FDA’s Oncology Center of Excellence Project Facilitate.