, by Shana Spindler
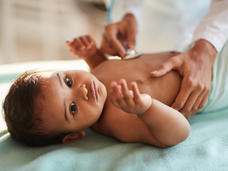
Adding the drug blinatumomab (Blincyto) to chemotherapy may help more infants with acute lymphoblastic leukemia (ALL) survive, according to the results of a small international clinical trial.
All 30 of the infants in the phase 2 study had ALL that was caused by specific changes involving the KMT2A gene, which are seen in about 80% of infants with ALL.
Overall, more than 90% of infants in the study who received the treatment combination as their initial treatment were still alive 2 years later. This survival rate is a large improvement over what’s been seen with chemotherapy alone in earlier studies.
The results were published April 27 in the New England Journal of Medicine.
The study addresses a critical need, according to the study team, for more effective treatments for infant ALL that is caused by changes in KMT2A, known as rearrangements. This form of ALL is aggressive across all age groups, particularly in infants, and is linked to poor outcomes. For example, historically, only about 40% of infants whose ALL has KMT2A rearrangements are still alive and disease free 5 years after diagnosis.
“This is a subgroup of patients who desperately need a better outcome from their disease,” said study leader, Inge van der Sluis, M.D., Ph.D., a pediatric oncologist and clinical pharmacologist at the Princess Máxima Center for pediatric oncology in Utrecht, the Netherlands.
Although researchers have tried using higher doses of chemotherapy, Dr. van der Sluis continued, “the prognosis for babies with KMT2A-rearranged ALL has not improved in recent decades.”
The finding is a “dramatic increase in the disease-free survival” for these infants, said Malcolm Smith, M.D., Ph.D., of NCI’s Cancer Therapy Evaluation Program, who was not involved in the study.
If this result can be confirmed in larger studies, he continued, it will be “an important advance for infants with KMT2A-rearranged ALL and a new standard therapy.”
Long-awaited improvements for a very poor prognosis
Blinatumomab, a type of immunotherapy called a BiTE, is a specially engineered antibody that “binds to leukemic cells on one side and to immune cells on the other side,” Dr. van der Sluis explained. “This connects the immune cells to the leukemic cells, and [the immune system] kills them.”
In 2017, the Food and Drug Administration approved blinatumomab to treat children and adults whose ALL had returned following at least one course of treatment. Subsequent clinical trials showed that blinatumomab substantially increased the percentage of children with ALL who were in remission compared with chemotherapy alone—and that blinatumomab caused fewer side effects.
Given the safety and efficacy of blinatumomab in both children and adults, Dr. van der Sluis was eager to test it in infants with KMT2A-rearranged ALL. The international study—which was funded in part by Amgen, the maker of blinatumomab—included 30 study participants, all of whom were younger than 1 year of age and had newly diagnosed KMT2A-rerranged ALL.
The patients were treated with a standard chemotherapy regimen known as the Interfant-06 protocol. After 1 month of chemotherapy, the infants all received one cycle of blinatumomab by continuous infusion through a vein for 4 weeks. After the blinatumomab infusion, the infants continued with standard chemotherapy treatments as needed.
Adding the 28-day treatment of blinatumomab to the standard chemotherapy treatment regimen led to a dramatic improvement in overall survival compared with what was seen in earlier studies.
For example, after a median follow-up of 2 years, overall survival in this group was 93%, compared with 66% in an earlier trial that used chemotherapy alone. And nearly 82% of the patients receiving blinatumomab remained alive without their disease returning during that time, a measure known as disease-free survival, compared with only 49% in the earlier trial.
This is a “spectacular improvement in outcome,” said Rob Pieters, M.D., Ph.D., of the Princess Máxima Center for pediatric oncology, the senior investigator on the study. He added that “the vast majority of relapses in this very aggressive type of leukemia occur during the first 2 years, so the data of this pilot study in 30 infants are very promising.”
The researchers noted that the side effects from blinatumomab therapy in the infants were similar to those seen in older patients and included fever, infection, high blood pressure, and vomiting. The most common serious side effect was a decrease in red blood cells, which occurred in five (17%) of the infants. Treatment was not halted for any of the study participants because of side effects.
Larger studies and longer follow-up are needed
Longer follow-up of the patients is awaited, the study authors noted. In addition, the Princess Máxima Center for pediatric oncology is sponsoring a larger study of 160 infants with newly diagnosed leukemia with KMT2A rearrangements, called Interfant-21, which has recently launched and will be open in 27 countries worldwide.
Questions remain around the most effective way to use blinatumomab, Dr. Smith said. In this particular study, infants received a single course of blinatumomab.
“Might two or three courses be more effective?” he wondered. Notably, in the Interfant-21 trial, a second cycle of blinatumomab will replace additional chemotherapy for most participants.
Harnessing the power of targeted therapies to reduce long-term side effects
Blinatumomab, which targets cancer cells specifically, is less toxic than the aggressive chemotherapy that is typically used to treat infants with ALL. This is an important advantage of blinatumomab, Dr. Smith noted.
“We expect that replacing chemotherapy with immunotherapy will lead to fewer side effects and more efficacious treatment,” said Dr. van der Sluis.
Dr. Pieters agreed. The “aim is to improve survival and reduce toxicity of [a] very intensive treatment,” he said.